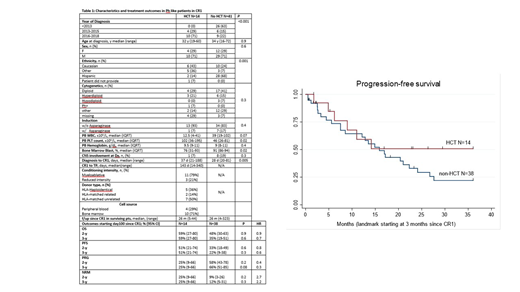
Introduction: Philadelphia (Ph) like acute lymphoblastic leukemia (ALL) is a high risk subtype of ALL, with the majority of patients overexpressing CRLF2; CRLF2 overexpression is associated with particularly poor outcomes (Jain, Blood, 2017). To date, the efficacy of hematopoietic cell transplantation (HCT) in these patients is unknown. Methods: In this retrospective study, we evaluated patients with CRLF2 overexpressed ALL who received or did not receive HCT. CRLF2 status was identified via FISH or multi-parameter flow cytometry. We identified 55 patients treated at our institution from 1992-2019 who had CRLF2 overexpression at diagnosis and achieved a first complete remission (CR1). To account for potential survival bias in the HCT group, outcomes between the two groups were compared in a landmark analysis starting at 3 months since CR1. Results: Baseline characteristics and treatment outcomes are described in Table 1. The median age was 32 years and 34 years, respectively, for HCT vs. non-HCT groups. In both groups, patients received high-intensity induction therapy with or without asparaginase. Patients who did not receive HCT were more likely (63%) to have been diagnosed prior to 2013, whereas all those treated with HCT were diagnosed after 2013. This difference reflects a change in practice at our institution after the description of CRLF2 overexpression as a poor prognostic factor. Median peripheral blood platelet count (102 k/uL vs 46 k/uL, p=0.02) and bone marrow blasts (76% vs 91%, p=0.02) at diagnosis were different between the HCT and non-HCT groups, respectively. In the HCT group, the majority of patients underwent myeloablative conditioning (n= 11, 79%) with a matched donor (n=9, 64%). With a median follow up of 26 months from CR1 in both groups, landmark analysis showed a trend for lower 3-year progression rate (25% vs 66%, HR=0.3, p=0.08) and improved progression-free survival (PFS) (51% vs 22%, HR=0.6, p=0.3) and overall survival (OS) (59% vs 35%, HR=0.6, p=0.3) in the HCT versus non-HCT groups. The median PFS was 16 months for the non-HCT group, and has not been reached for the HCT group. In the HCT group, PFS appears to have reached a plateau at 14 months, with 6 of 14 patients remaining alive in remission at a median follow-up of 24 months (range 17-41). Conclusions: CRLF2 overexpression in ALL is associated with a high rate of progression. Allogeneic HCT is beneficial against relapse, showing a trend for improved PFS and OS.A larger sample size and longer follow up is needed to confirm these findings.
Popat:Bayer: Research Funding; Incyte: Research Funding; Jazz: Consultancy. Oran:AROG pharmaceuticals: Research Funding; Astex pharmaceuticals: Research Funding. Qazilbash:Bioclinical: Consultancy; Genzyme: Other: Speaker; Amgen: Consultancy, Other: Advisory Board; Autolus: Consultancy. Ciurea:Miltenyi: Research Funding; Spectrum: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Kiadis Pharma: Membership on an entity's Board of Directors or advisory committees, Other: stock holder. Jain:BMS: Research Funding; Cellectis: Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jabbour:Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding. Kantarjian:Astex: Research Funding; Takeda: Honoraria; BMS: Research Funding; Ariad: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Agios: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; Cyclacel: Research Funding. Konopleva:Astra Zeneca: Research Funding; Kisoji: Consultancy, Honoraria; Ablynx: Research Funding; Agios: Research Funding; Calithera: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding. Kebriaei:Pfizer: Honoraria; Kite: Honoraria; Amgen: Research Funding; Jazz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal