Background:Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutations in acute myeloid leukemia (AML) are a common indication for allogeneic hematopoietic stem cell transplant (HCT) in first complete remission (CR1). Despite HCT, relapses are common and cure rates are limited thereafter. The use of FLT3 inhibitors as post-HSCT maintenance therapy has not been prospectively evaluated in the phase 3 setting. Gilteritinib is a selective, potent FLT3 inhibitor with robust activity and favorable tolerability in relapsed/refractory AML. This trial will compare the safety and efficacy of 2-year maintenance therapy with gilteritinib versus placebo in patients with FLT3-ITD+ AML in CR1 after allogeneic HSCT.
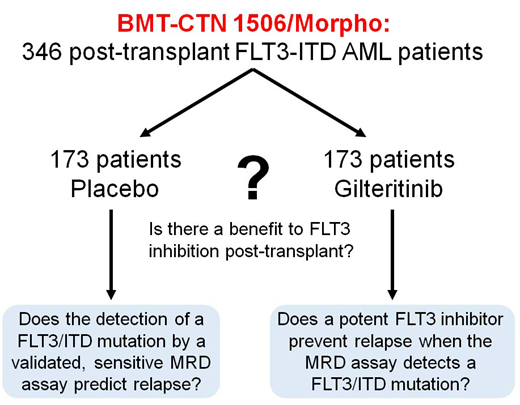
The broad goal of this study is to determine if there is a benefit to FLT3 inhibition as maintenance therapy post-HCT and to identify which patients, if any, benefit. We will use a novel NGS-based assay for FLT3-ITD mutations to detect minimal residual disease (MRD) in order to stratify patients pre-HSCT and correlate with relapse post-HCT.
Study Design and Methods: This Phase 3, randomized, double-blind, placebo-controlled multicenter trial (NCT02997202; Blood and Marrow Transplant Clinical Trials Network Protocol 1506), is being conducted at 149 sites worldwide. The target enrollment is 532 adult subjects (aged ≥18 years) with FLT3-ITD+ AML in CR1 who are ≥30 days and ≤90 days from scheduled allogeneic HSCT. Of these 532 subjects, 346 subjects who have achieved successful engraftment without uncontrolled graft-versus-host disease (GVHD) or other serious toxicity will be randomized (1:1; stratified by conditioning regimen intensity, time from HSCT [Day 0] to randomization [30-60 days vs 61-90 days], and presence of minimal residual disease [MRD] in the pre-transplant bone marrow sample) to receive oral gilteritinib (120 mg) or matching placebo as maintenance therapy for 2 years. The primary endpoint is relapse-free survival (RFS) in the two treatment arms; RFS will be assessed from the time of randomization to the time of death or morphologic leukemia relapse (as defined by Revised IWG criteria). MRD status will continue to be monitored over the duration of the maintenance therapy, although investigators will be blinded to the MRD assay results. Overall survival is a key secondary endpoint. Other endpoints include safety/tolerability, non-relapse mortality, event-free survival, incidences of acute/chronic GVHD, and MRD. As of July 31, 2019, 341 patients have been registered and 236 have been randomized.
To achieve a target number of 346 subjects for randomization (n=173 per treatment arm), we estimated that enrollment of 532 subjects would be required given an expected dropout rate of 35% between the time of registration to the time of randomization. The RFS rate in the placebo arm (control) is assumed to be 67% at 1 year, 59% at 2 years, and 55% at 3 years (according to the Center for International Blood & Marrow Transplant Research data for FLT3-ITD+ patients transplanted in CR1 who were alive and were progression free at 60 days). A total of 122 events will provide 85% power to detect a hazard ratio (HR) of 0.57 (corresponding to a 15% difference in 2-year RFS) with two-sided significance level of 0.05. With a 2-year accrual period and a 5% annual dropout rate, enrollment of 346 subjects will ensure a high probability of obtaining 122 events. The primary efficacy analysis will be performed using the intention-to-treat (ITT) population, which is defined as all subjects who are randomized. RFS will be compared across the treatment groups using the stratified Log-rank test. A stratified Cox model with treatment as covariate will be used to determine HR and confidence intervals at 1, 2, and 3 years. Kaplan-Meier estimates of RFS will be reported at 1, 2, and 3 years. No interim efficacy or futility analyses are planned.
Levis:Daiichi Sankyo Inc: Consultancy, Honoraria; Menarini: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Astellas: Consultancy, Research Funding; FUJIFILM: Consultancy, Research Funding. Hamadani:Otsuka: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Medimmune: Consultancy, Research Funding; Pharmacyclics: Consultancy; Takeda: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau; Celgene: Consultancy; Merck: Research Funding; Janssen: Consultancy. Rosales:Astellas: Employment. Delgado:Astellas: Employment. Bahceci:Astellas: Employment, Patents & Royalties. Devine:Kiadis Pharma: Other: Protocol development (via institution); Bristol Myers: Other: Grant for monitoring support & travel support; Magenta Therapeutics: Other: Travel support for advisory board; My employer (National Marrow Donor Program) has equity interest in Magenta. Horowitz:Actinium: Other: Unrestricted educational and research grant; Gamida Cell: Other: Unrestricted educational and research grant, Research Funding; Oncoimmune: Other: Unrestricted educational and research grant; Miltenyi Biotech: Other: Unrestricted educational and research grant, Research Funding; Janssen: Other: Unrestricted educational and research grant, Research Funding; Kite Pharma/Gilead: Other: Unrestricted educational and research grant, Research Funding; Magenta: Consultancy, Other: Unrestricted educational and research grant; GlaxoSmithKline: Other: Unrestricted educational and research grant; Shire: Other: Unrestricted educational and research grant; CSL Behring: Other: Unrestricted educational and research grant, Research Funding; Seattle Genetics: Other: Unrestricted educational and research grant; Mesoblast: Other: Unrestricted educational and research grant, Research Funding; Bristol-Myers Squibb: Other: Unrestricted educational and research grant, Research Funding; Daiichi Sankyo: Other: Unrestricted educational and research grant; Sanofi: Other: Unrestricted educational and research grant, Research Funding; Pharmacyclics: Other: Unrestricted educational and research grant; Amgen: Other: Unrestricted educational and research grant; Chimerix: Other: Unrestricted educational and research grant; Regeneron: Other: Unrestricted educational and research grant. Chen:Incyte: Consultancy; Kiadis: Consultancy; Magenta: Consultancy; Abbvie: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal