Introduction: Neurologic toxicities have resulted in restrictions in driving/engaging in hazardous occupations and activities for pts receiving marketed T-cell-engaging CD19-directed bispecific antibodies and chimeric antigen receptor T-cell (CAR-T) therapies. Mosunetuzumab (M; RG7828) is a full-length, fully humanized immunoglobulin G1 (IgG1) bispecific antibody targeting both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). Clinical trials investigating the safety and preliminary efficacy of M currently mandate a driving restriction for enrolled pts while receiving treatment. Data collected to date show that neurologic toxicities associated with M may be milder than for other CD19-directed therapies, which may be due in part to the step-up dosing regimens investigated (Budde et al. ASH 2018; Bartlett et al. ASCO 2019). We initiated a systematic review of neurologic adverse events (NAEs) in pts treated with M to characterize the nature of driving-impacting NAEs and to develop a more patient-centric approach to NAE risk-mitigation that would reduce the burden on pts in M clinical trials.
Methods: A systematic review of NAEs observed in the ongoing Phase I/Ib study (GO29781; NCT02500407) of M in relapsed or refractory non-Hodgkin lymphoma (NHL) pts (n=205) was performed (data cutoff: Jan 31, 2019; median safety follow-up: 116 days). NAEs were defined as any AE reported as a Preferred Term (PT) within the System Organ Class (SOC) Nervous System Disorders and SOC Psychiatric Disorders, as recommended by FDA. NAEs were further classified by those that have impact on cognition and consciousness (CC-NAEs), and those that may impair driving (DI-CC-NAEs). In conjunction with an independent neurology consultant, DI-CC-NAEs were adjudicated based on medical judgement and determined a priori based on reported PTs. Univariate and multivariate logistic regression with a 5% significance level was performed to determine the association of demographic and disease characteristics with DI-CC-NAEs occurring in the first 3 cycles of M treatment, as 90% (27/30) of events occurred within this period.
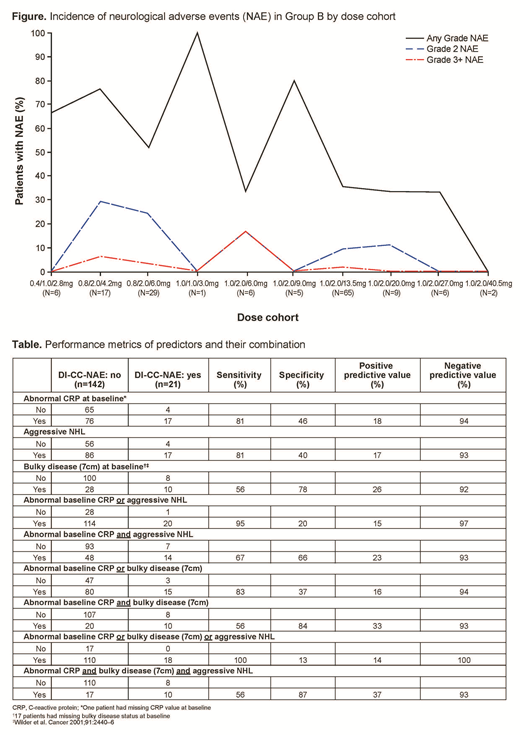
Results: NAEs were observed in 49% (100/205) of enrolled pts and were mostly grade (Gr) 1-2 (Gr 1, 69/205 [34%]; Gr 2, 24/205 [12%]). The most frequent NAEs were headache (32/205, 16%), dizziness (27/205, 13%), and insomnia (19/205, 9%). Gr ≥3 NAEs were rare (3%, all Gr 3) and occurred as clinical sequelae to non-NAEs (e.g. Gr 3 hepatic encephalopathy secondary to Gr 4 liver function test increased, Gr 3 seizure secondary to electrolyte disturbance, Gr 3 post-herpetic neuralgia). The majority of NAEs occurred early and were transient (median onset from first M infusion: 16 days; median duration: 10 days). Most resolved (11% were unresolved at time of data cut). Frequency of NAEs was not associated with M dose level (Figure) or exposure (Li et al. ASH 2019). At the time of data cut, 30 DI-CC-NAEs (Gr 1, 17/30 [57%]; Gr 2, 9/30 [30%]; Gr 3, 4/30 [13%]) were reported, including PTs of confusional state, delirium, disturbance in attention, encephalopathy, hallucination, memory impairment, and seizure. These events were observed in 24 (12%) pts, and were mostly Gr 1-2 (26/30, 87%). Three pts experienced four Gr 3 DI-CC-NAEs (confusional state, hepatic encephalopathy, encephalopathy, seizure). The majority of DI-CC-NAEs (67%) occurred in pts with aggressive NHL (aNHL) and who had elevated C-reactive protein (CRP) at baseline based on the local institutional upper limit of normal (sensitivity: 67%; specificity: 66%; positive predictive value: 23%; negative predictive value: 93%; Table). Other characteristics included in the multivariate analysis were age, sex, and tumor bulk, but these did not demonstrate association with occurrence of DI-CC-NAEs.
Conclusion: Based on these analyses, the driving restriction that applied to all pts for the duration of M treatment was amended to a restriction covering the first 2 cycles only in a subset of pts who had aNHL and elevated CRP at baseline. This novel approach of individualized risk mitigation for driving based on in-depth assessment of NAEs allows for a more targeted and patient-centric mitigation strategy to optimize the protection of pts at risk, and is useful during clinical development when the safety profile is being characterized. As further data are accumulated, the multivariate analysis can be updated to further refine the pts at risk for DI-CC-NAE.
Diefenbach:Bristol-Myers Squibb: Consultancy, Research Funding; Denovo: Research Funding; Genentech: Consultancy, Research Funding; Incyte: Research Funding; LAM Therapeutics: Research Funding; MEI: Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Millenium/Takeda: Research Funding; Trillium: Research Funding. Assouline:Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau. Bosch:Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd/Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Cheah:F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; AbbVie: Research Funding; Celgene: Research Funding; Gilead: Honoraria; Janssen: Honoraria; Acerta: Honoraria; Loxo: Honoraria. Kim:J + J: Research Funding; Novartis: Research Funding; Celltrion: Research Funding; Novartis: Research Funding; Donga: Research Funding; Kyowa-Kirin: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding. Matasar:Merck: Consultancy, Equity Ownership; Genentech, Inc.: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Bayer: Consultancy, Honoraria, Other; Roche: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Seattle Genetics: Consultancy, Honoraria, Other: Travel, accomodation, expenses, Research Funding; Rocket Medical: Consultancy, Research Funding; Teva: Consultancy; Bayer: Other: Travel, accommodation, expenses; Janssen: Honoraria, Research Funding; Juno Therapeutics: Consultancy. Panizo:Janssen: Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Speakers Bureau. Yoon:F. Hoffmann-La Roche Ltd: Research Funding. Bender:Genentech, Inc.: Employment, Equity Ownership. Hernandez:Genentech, Inc.: Employment, Equity Ownership. Li:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. McCall:F. Hoffmann-La Roche Ltd: Equity Ownership; Genentech, Inc.: Employment, Equity Ownership. O'Hear:Genentech, Inc.: Employment; F. Hoffmann-La Roche Ltd: Equity Ownership. Wei:Genentech, Inc./F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Yin:Genentech, Inc: Employment, Equity Ownership. Yousefi:F. Hoffmann-La Roche Ltd: Employment. Kwan:Genentech, Inc: Employment, Equity Ownership. Nastoupil:Celgene: Honoraria, Research Funding; Bayer: Honoraria; Spectrum: Honoraria; Janssen: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Novartis: Honoraria; TG Therapeutics: Honoraria, Research Funding; Gilead: Honoraria.
Mosunetuzumab (RG7828) is a full-length, fully humanized immunoglobulin G1 (IgG1) bispecific antibody targeting both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). Mosunetuzumab redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal