Background: The advent of oral-targeted drugs has improved treatment outcomes for patients (pts) with CLL. Nonetheless, some pts prove intolerant or resistant to therapy and/or fail to achieve complete response (CR) with uMRD. Liso-cel is an investigational, anti-CD19, defined composition, 4-1BB CAR T cell product administered at a target dose of CD4+ and CD8+ CAR T cells. TRANSCEND CLL 004 is an open-label phase 1/2 study of liso-cel in pts with R/R CLL/SLL (NCT03331198).
Methods: Eligible pts with CLL/SLL had received at least 3 (standard-risk disease) or at least 2 (high-risk disease: del[17p], TP53 mutation, unmutated IGHV, or complex karyotype) prior lines of therapy, including a Bruton's tyrosine kinase inhibitor unless contraindicated. Pts with active untreated central nervous system disease, Eastern Cooperative Oncology Group performance status >1, or Richter's transformation were excluded. After 3 days of lymphodepletion with fludarabine/cyclophosphamide, pts received liso-cel infusion; 2 dose levels (DLs) were tested: DL1=50×106 and DL2=100×106 CAR+ T cells. Dose-limiting toxicities (DLTs) were evaluated for 28 days postinfusion. Responses were assessed by 2018 International Workshop on CLL criteria. MRD was assessed at a sensitivity of ≤10-4 in blood and/or bone marrow (BM) lymphocytes. Liso-cel CAR T cells were monitored by flow cytometry of blood cells from treated pts over time. Serum cytokines and chemokines were assessed via an electrochemiluminescence platform.
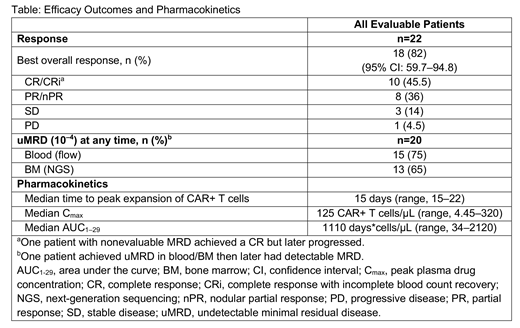
Results: At data cutoff, 23 pts were evaluable for safety and 22 for efficacy. Median age was 66 (range, 49‒79) years; 83% (19/23) of pts had high-risk disease. Pts had a median of 5 (range, 2‒11) prior therapies. All pts had received prior ibrutinib; 56.5% (13/23) had progressed on ibrutinib and received therapy with venetoclax; 91% (21/23) were refractory to, or had relapsed on, ibrutinib; and 9% (n=2) were intolerant to ibrutinib. Liso-cel was successfully manufactured in 96% of pts, with the established process. Nine pts were treated at DL1 and 14 pts at DL2. Two pts had DLTs (all at DL2: grade 4 hypertension in 1 pt; grade 3 encephalopathy, grade 3 muscle weakness, and grade 4 tumor lysis syndrome in 1 pt). The most common grade 3/4 treatment-emergent adverse events were thrombocytopenia, 70%; anemia, 96%; neutropenia, 56.5%; leukopenia, 43.5%. Two pts had grade 3 cytokine release syndrome (CRS); 5 pts had grade ≥3 neurological events (NEs), including encephalopathy (n=3). Median time to onset of CRS and NE was 4 (range, 1-10) days and 4 (range, 2-21) days, respectively. The median duration of CRS and NE was 5 (range, 2-30) days and 21 (range, 6-169) days, respectively. To manage CRS and/or NE, 61% (n=14) of pts received tocilizumab and 48% (n=11) received corticosteroids. Lymph node tumor burden correlated with NE (P=0.025). Additional disease distribution correlates are being evaluated and will be presented. Cytokine analyses revealed that NE onset was preceded by elevated TNFα and IL16 early after liso-cel infusion (P<0.01). The table shows best responses at median follow-up of 9 months. Among evaluable pts, the best overall response rate was 82%; the best CR/CRi rate was 45.5%. Sixty-eight percent (15/22) of pts achieved objective response by Day 30; 78% (7/9) of responders with ≥9 months of postdose follow-up have remained progression-free. In 6 pts, responses deepened over time (3 from partial response [PR] to CR, 2 from stable disease [SD] to PR, and 1 from SD to CR). Among 20 pts evaluable for MRD, most achieved blood and/or BM uMRD (Table); 60% (12/20) achieved BM uMRD by Day 30. All pts who achieved blood and/or BM uMRD have remained uMRD to date.
Conclusions: In this population of heavily pretreated pts with R/R CLL/SLL (all received prior ibrutinib and half failed both prior venetoclax and ibrutinib), liso-cel toxicities, including CRS and NE, were manageable at both DLs tested. Objective responses, CRs, and uMRD were rapidly achieved, with durable responses past 6 months. Updated safety, pharmacokinetic, and efficacy results from the phase 1 monotherapy part of the study will be reported. The phase 2 portion of the study is currently enrolling at DL2.
Siddiqi:Kite, A Gilead Company: Research Funding; TG Therapeutics: Research Funding; Celgene: Research Funding; Janssen: Speakers Bureau; Seattle Genetics: Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; PCYC: Consultancy, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Other: travel support, Research Funding; BeiGene: Research Funding. Soumerai:BeiGene: Research Funding; BostonGene: Research Funding; AbbVie: Consultancy; Verastem: Consultancy; Genentech/Roche: Research Funding; TG therapeutics: Research Funding. Dorritie:Juno: Research Funding. Stephens:Karyopharm: Research Funding; Gilead: Research Funding; Acerta: Research Funding. Riedell:Bayer: Honoraria, Speakers Bureau; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Arnason:Regeneron Pharmaceuticals, Inc.: Consultancy; Celgene/Juno: Consultancy. Kipps:Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Velos-Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jannsen Pharmaceutical Companies of Johnson & Johnson: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca, Inc.: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees. Gillenwater:Juno Therapeutics, a Celgene Company: Employment, Equity Ownership. Gong:Celgene: Employment, Equity Ownership. Dubovsky:Celgene: Employment. Rytlewski:Juno Therapeutics, a Celgene Company: Employment, Equity Ownership; Adaptive Biotechnologies: Equity Ownership. Yang:Juno Therapeutics, a Celgene Company: Employment. Wierda:AbbVie: Research Funding; Genentech: Research Funding; Pharmacyclics LLC: Research Funding; Acerta Pharma Inc: Research Funding; Gilead Sciences: Research Funding; Xencor: Research Funding; Juno Therapeutics: Research Funding; Sunesis: Research Funding; KITE pharma: Research Funding; Oncternal Therapeutics Inc.: Research Funding; Miragen: Research Funding; GSK/Novartis: Research Funding; Cyclcel: Research Funding; Janssen: Research Funding; Loxo Oncology Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal