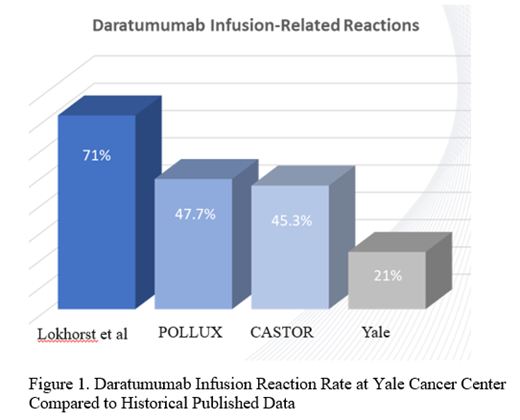
Background: Multiple myeloma (MM) is an incurable and heterogeneous neoplasm of clonal plasma cells. Fortunately, the introduction of novel therapeutic agents has altered the landscape of MM with resultant improvements in both overall survival and quality of life for this patient population. The CD38-targeted humanized monoclonal antibody, daratumumab, has played a monumental role in this advancement. It has yielded significantly increased overall response rates and progression-free survival in landmark trials for relapsed/refractory MM (RRMM) (Dimopoulos MA et al, 2018; Spencer A et al, 2018) and has also now been successful in the induction therapy setting for both transplant-ineligible (Mateos MV et al, 2018; Facon T et al, 2019) and transplant-eligible (Moreau P et al, 2019) patients. However, concern has been raised regarding the high incidence of infusion-related reactions, reported to be as high as 71% in original phase I-II trials of daratumumab monotherapy (Lokhorst et al, 2015). We describe the real-world practice pattern of daratumumab utilization at Smilow Cancer Hospital, demonstrating an excellent safety profile and tolerability among our patients.
Methods: We retrospectively reviewed the use of daratumumab in the management of RRMM for 57 patients treated at Smilow Cancer Hospital. Data was collected on the choice of treatment regimen, number of cycles administered, and the number and grade of infusion reactions. Per institutional protocol, patients receiving an initial cycle of daratumumab therapy were administered hypersensitivity premedication with acetaminophen, diphenhydramine, dexamethasone, and montelukast.
Results: A total of 57 patients receiving treatment with daratumumab were evaluated and received an average of 10.3 cycles with a median of 8 cycles (range, 2-35 cycles). Of these 57 individuals, only 21.1% (12/57) experienced an infusion reaction all of which were grade 1-2 in severity. One hundred percent (12/12) of these patients developed reactions during the first infusion of daratumumab and all individuals completed the dose with no subsequent reactions for the remainder of their treatment course. All patients were able to be switched to rapid daratumumab infusion by their third dose. Of the evaluable patients, 5.4% (3/56) received daratumumab monotherapy while the remaining 94.6% (53/56) were treated with 3 or 4 drug combination regimens. At the time of this report, 49.1% (26/53) of our patients are still receiving daratumumab as a part of their anti-plasma cell therapy.
Conclusions: Immunotherapy has served as a large contributor to the dramatic improvement observed in the management of patients with MM. Our review of real-word experience with use of daratumumab demonstrates that this agent is well-tolerated with infusion reaction rates that are significantly lower than those previously reported in the literature (Figure 1). The universal incorporation of the leukotriene receptor antagonist, montelukast, as premedication at our institution may have contributed to these enhanced outcomes. An expanded, multi-center study is being launched to evaluate a real-world experience of adverse event profile and patient-reported quality of life measures with daratumumab utilization, given the paucity of such data currently available.
Anderson:Celgene: Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Janssen: Speakers Bureau. Neparidze:Janssen Scientific Affairs, LLC: Research Funding; Eidos Therapeutics: Other: Member of Independent Diagnostic Committee; MMRF/Synteract: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal