Background:
Autologous hematopoietic stem cell transplantation (ASCT) is the current standard of care in patients with multiple myeloma (MM) to support reconstitution of the bone marrow after myeloablative chemotherapy. Mobilization of blood progenitor cells to the peripheral circulation is stimulated via administration of granulocyte colony-stimulating factor (G-CSF) with or without chemotherapy. The addition of chemotherapy, typically cyclophosphamide, increases the yield of peripheral blood stem cells (also known as CD34+ cells) at the expense of additional toxicity. The unpredictability of harvest yield and the potential need for weekend apheresis sessions complicates mobilization planning when using chemotherapy. Plerixafor, a CXCR4 antagonist, in combination with G-CSF has been shown to be superior to G-CSF alone, leading to higher CD34+ yields in fewer apheresis sessions [Giralt et al, 2014]. However, the advantage of the combination of plerixafor with G-CSF is less well described in the context of new treatment options that can impair stem cell harvest yields when using G-CSF as the sole mobilization agent.
Objective:
The primary objective of this study was to determine clinical outcomes associated with G-CSF alone vs G-CSF + chemomobilization vs G-CSF + plerixafor from initiation of mobilization until 6 to 12 months after ASCT.
Method:
This is a retrospective study in patients with MM, carried out at 10 centers in the US. Patients were eligible if ≥ 18 years and undergoing first ASCT (≤ 12 months from initial diagnosis and no disease progression). Eligible patients were identified through a database query of hospital records of oncology patients diagnosed with MM and eligible for ASCT before June 2016. Patients fulfilling inclusion and exclusion criteria were attributed to three treatment arms depending on the mobilization regimen received:
Arm 1: G-CSF + plerixafor
Arm 2: G-CSF + chemomobilization
Arm 3: G-CSF
Patients will be selected until 170 patients are assigned to each treatment arm or patient records are exhausted. Demographics, induction regimen, mobilization regimens and outcomes, apheresis, transplantation outcomes and survival status were extracted from medical charts and entered in the electronic case report form. Here, we present interim results from data collected at three centers as of June 24, 2019.
Results:
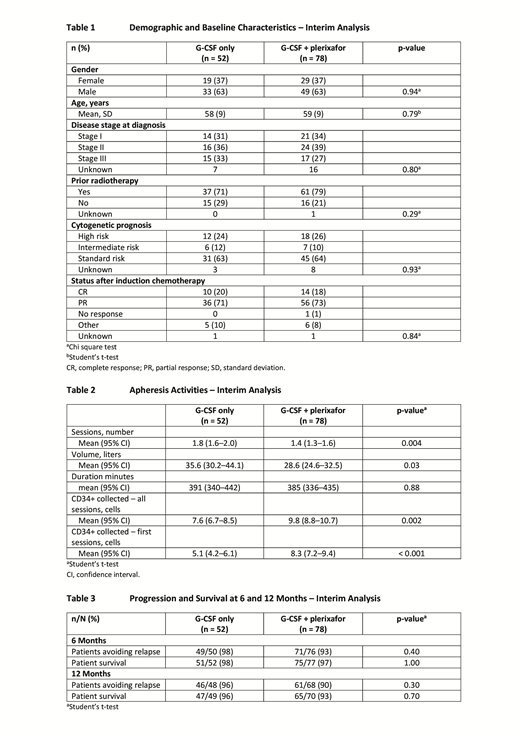
At the interim analysis cut-off date, 130 patients were included in the database (G-CSF: 52; G-CSF + plerixafor: 78; G-CSF + chemomobilization: 0). Demographic and baseline characteristics were comparable between the two treatment arms. More male patients were included (male: 63%, female: 37%) and the mean age was 58 and 59 years in the G-CSF and G-CSF + plerixafor groups, respectively (Table 1).
Disease stage and the proportion of patients with poor-, intermediate- and high-cytogenetic risk status were similar in the two treatment groups. Few patients had a complete response (CR) after chemotherapy, but response rate (CR + partial response) was 91% in both treatment arms.
The burden of apheresis in terms of number of sessions and blood volume processed was lower in patients receiving G-CSF + plerixafor compared with patients receiving G-CSF only (Table 2).
CD34+ yield was higher in the G-CSF + plerixafor treatment arm, especially after the first apheresis session (Table 2). There was no difference in ASCT outcome between the two treatment groups. All patients had neutrophil engraftment except for one patient in the G-CSF only group, and all patients had platelet engraftment except one patient in each treatment group.
There was no significant difference in the proportion of patients experiencing relapse between the two treatment groups at 6 and 12 months of follow-up. Overall, ≥ 90% of patients avoided relapse at 12 months. 96% and 93% of patients were still alive at 12 months in the G-CSF only and G-CSF + plerixafor treatment groups, respectively (Table 3). For the purpose of this study, safety data was not reviewed [Mozobil PI, 2019].
Conclusions:
Per interim results, plerixafor reduced the burden of apheresis and increased CD34+ yields. Transplantation outcomes, relapse and overall survival at 6 and 12 months were not significantly different between the two treatment arms.
Therefore, plerixafor could increase apheresis efficiency without compromising ASCT success in the context of currently used chemotherapy regimens.
Mark:Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Takeda: Honoraria. Bubalo:Sanofi: Other: Research and writing support. Barnes:Sanofi: Employment. Drea:Sanofi-Genzyme: Employment. Fausel:Sanofi-Genzyme: Other: Grant to cover costs of data collection.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal