Introduction
In most cases, haploidentical stem cell transplantation (haplo-HSCT) with negative depletion of α/β(+) T cells and CD19+ B lymphocytes from the graft is used to treat pediatric patients. The results are promising. However, the results of this method in adult patients is controversial. The accumulation of experience in haplo-HSCT with TCRαβ / CD19 + depletion in the group of adult patients is relevant.
Aim
Evaluation of the effectiveness and characterization of the most frequent complications in adult patients with hematological malignancies who underwent allo-HSCT from a haploidentical donor with depletion of TCRαβ/CD19 + cells.
Patients and methods
The analysis included 32 patients (14 males/18 females) with acute myeloid leukemia (AML, n=12), acute lymphoblastic leukemia (ALL, n=11), myelodysplastic syndrome (MDS, n = 6), chronic myeloid leukemia (CML, n = 1), primary myelofibrosis (PMF, n = 1), lymphoproliferative disease (LPD, n = 1). Median age was 28 years (range, 17-58). Disease status of acute leukemia at the beginning of pre-transplant conditioning was first complete remission (CR1) in 14 patients, CR2 in 7 and active disease in 2 patients. Pre-transplant conditioning regimen: RIC (Treosulfan 42 g/m2, Melphalan 140 mg/m2, Fludarabine 150mg/m2), MAC (Treosulfan 42 g / m2, Thiophosphamide 10 mg / kg, Fludarabine 150 mg / m2). Immunosuppressive therapy: Rituximab, Bortezomib, Tocilizumab, Abatacept. Immunomagnetic separation was performed using a CliniMACS Plus device. Descriptive statistics methods were used for analysis. The probabilities of survival and graft versus host disease (GVHD) rate were estimated using the Kaplan-Meier method.
Results
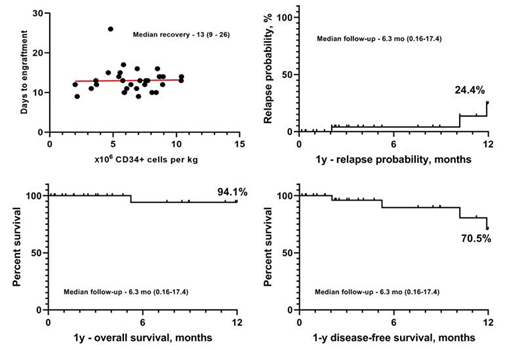
Log of TCRαβ + depletion was 1.61-5.33 (Me = 3.66). The median dose of CD34+ cells in transplant was 6.8 * 106/kg (range, 2.0-10.8). The median time to white blood cells recovery was +13 days after haplo-HSCT (range, 9-26). Median follow-up was 6.4 months. Transplant related mortality was 3.1%. Primary engraftment - 96.8%. Graft hypofunction - 16.2%. The probabilities of overall and disease-free survival for 12 months were 94.1% and 70,5%, respectively. The probability of relapse was 24.4% (Fig. 1). The probability of developing acute GVHD was 25%, GVHD rate was 18,75% including grade I (n=1), grade II (n=2), grade III (n=3) (data not shown). In 4 cases complete response was achieved with administration of first line immunosuppression therapy. 1 case (grade III GVHD) required administration of second line immunosuppression therapy - methylprednisolone, and the response was complete. 1 patient developed chronic GVHD. Nonclassical infectious complications prevail: viral infections (CMV, HHV6 and EBV) was 58.8%, fever with an unverified infectious agent was 15.6%, and tuberculosis in two cases (6.2%). Immunological events not associated with GVHD - 21.8% (TMA, TTP, myasthenia gravis, AIHA, PRCA).
Conclusion
In some cases, haplo-HSCT is the only HSCT option for an adult patient. The frequency of viral infectious complications and relapses in adult patients after haplo-HSCT TCRαβ / CD19 + depletion is comparable to the results in the pediatric population. Haplo-HSCT with TCRαβ / CD19 + depletion is characterized by minimal toxicity and a short period of myelotoxic agranulocytosis. Among the undesirable phenomena in the first place are infectious complications and frequent immunological events that do not fit into the criteria for GVHD, but affect the patient's quality of life and length of hospital stay.
Maschan:Miltenyi Biotec: Other: lecture fee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal