Background: Current guidelines recommend the use of antifungal prophylaxis during, at least, the first three months post allogeneic stem cell transplantation (allo-SCT). However, these drugs are not exempt of adverse effects due to their own toxicity or drug-drug interactions. In addition, breakthrough mold infections seem to be emerging as a new problem in this setting.
Objectives: The aim of this study was to analyze the benefit of a strategy in which allo-SCT patients were not routinely prescribed antifungal drugs except for the case of the development of neutropenic fever and/or exposure to high dose of corticosteroids. Main objectives were 1) requirement and time of exposure to antifungal treatment and 2) cumulative incidence (CI) of proven or probable IFI at 1, 3 and 6 months after transplant. Other objectives were IFI description and overall survival (OS).
Patients and methods: 319 patients who underwent a first allo-SCT at our center between 2010 and 2017 and were not under antifungal prophylaxis at the moment of transplant were included. 181 patients (57%) were males and median age was 51 years, range (4-74). Acute myeloid leukemia (35%) was the most frequent diagnosis and myeloablative conditioning regimen was mostly used (69%). 99 patients (31%) received the stem cells from a mismatched donor and BM was the preferred stem cell source (72%). Fourteen patients (4%) did not engraft. The median length of neutropenia <0.5x109/L and <0.1x109/L were 12 days and 6 days, respectively. Forty-two patients (14%) developed acute GVHD grade III-IV. Post-mortem study was performed in 28 of the deceased patients (43%).
Results:Thirty-one 31 patients (9.7%) did not receive antifungal drugs along the course of the transplantation, 58 patients (18%) required it for less than 1 week and only 24% were on therapy more than 3 weeks. The median total days on antifungal drugs was 13 days (0-175) for the entire population and 11 days (0-109) in patients who did not develop fungal infection and/or acute GVHD II-IV.
Fluconazole (61%) was the main antifungal drug used during the neutropenic phase, followed by caspofungin (27%). Time on fluconazole was significantly shorter than caspofungin, 8 vs. 11 days, p=0.001. A change on antifungal therapy was needed in 130 (45%) patients and was more frequent in those who were receiving fluconazole (57%) compared to those who were receiving caspofungin (20%), p<0.0001.
The CI of proven or probable IFI at day 30, 90 and 180 after transplant was 2.5%, 5.1% and 5.5%, respectively. Very importantly, none of the 106 patients who did not receive antifungal drugs or received less than one-week developed IFI. Twelve mold infection, 4 in the postmortem study, and 5 yeast infection were diagnosed. Microbiological identification was obtained in 9 patients; C. parapsilosis (3), Rhizopus (2), C. orthopsilosis (1), Criptococo laurentii (1), A. fumigatus (1), Alternaria spp. (1).
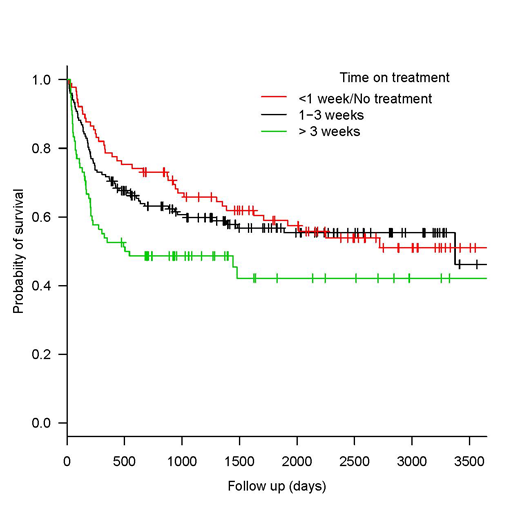
The restricted administration of antifungal drugs did not affect the overall survival, Figure 1.
Conclusion: In our experience, a restrictive strategy for the use of antifungal drugs in the setting of allo-SCT is feasible and effective. It results in similar cumulative incidences of fungal infection than the recommended policy. Moreover, it allows almost 10% of patients to remain free of antifungal treatment throughout the procedure and one fourth of them being exposed for less than a week. This data supports the change in the antifungal prophylactic paradigm for allo-SCT patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal