Although the thymus has a remarkable capacity for repair following acute injury, such as that caused by the conditioning required for successful hematopoietic cell transplant (HCT), the mechanisms underlying this endogenous regeneration remain poorly understood. Delayed T cell reconstitution occurs following thymus insult and can exceed more than a year post-transplant due to a delay in full recovery of thymic output, function and T cell repertoire. Therefore, strategies to enhance T cell reconstitution post-transplant represents a rational approach to significantly improve the overall outcome of allo-HCT. We propose that enhancing thymic function will boost T cell reconstitution and substantially increase immune responses following allo-HCT. Our recent studies have identified two critical pathways that govern thymic regeneration; centered on secretion of BMP4 by endothelial cells (ECs) and IL-22 by innate lymphoid cells (Dudakov 2012 Science 336:91; Dudakov 2017 Blood 130:933; Wertheimer 2018 Sci Immunol 3:19). However, the specific regulatory mechanisms that trigger these regeneration-associated factors (RAFs) after damage remain unclear. Given that our prior work revealed that the presence of DP thymocytes suppresses the production of RAFs like IL-23, a key downstream mediator of IL-22; and the high basal rate of thymocyte apoptosis, as apoptotic thymocytes form the bulk of developing T cells, we hypothesized that apoptotic DP thymocytes were mediating this suppression of RAFs under homeostatic conditions. Upon injury, loss of DP thymocytes leads to reduced apoptotic signaling and reduced suppression of RAFs, triggering thymic recovery (Fig 1A).
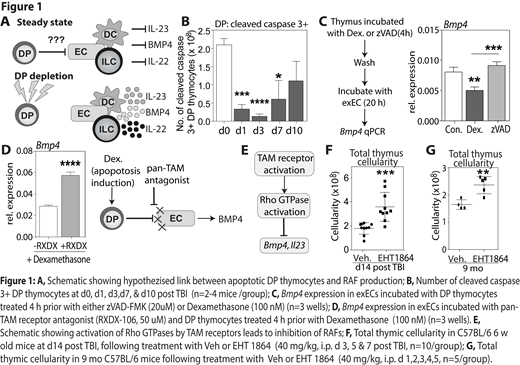
Consistent with this hypothesis, our preliminary data shows a significantly reduced number of apoptotic thymocytes after total body irradiation (TBI, 550 cGy), as measured by cleaved caspase 3 levels (Fig 1B). Additionally, co-culture of apoptotic thymocytes results in reduced Bmp4 expression in ECs, which is rescued by inhibition of thymocyte apoptosis using the pan-caspase inhibitor zVAD-FMK (Fig 1C). One way in which apoptotic thymocytes could induce this suppression of RAFs is via TAM receptor activation, which is supported by our data demonstrating increased Bmp4 expression in ECs treated with a pan-TAM receptor antagonist and subsequently co-cultured with apoptotic thymocytes (Fig 1D). Interestingly, TAM receptors can activate Rac1, a Rho GTPases involved in actin cytoskeletal rearrangement; converging neatly on our previous data showing that inhibition of Rac1 with small molecule inhibitors led to robust induction of Bmp4 and Il23 expression. Therefore, we propose that in steady-state, apoptotic thymocytes activate TAM receptors on ECs and DCs and induce intracellular activation of Rac1, which ultimately suppresses the production of BMP4 and IL-23; but after damage, when the number of apoptotic thymocytes drops precipitously, this suppression is abrogated, allowing for thymic regeneration (Fig 1E). Importantly, we demonstrate here that this pathway can be therapeutically targeted, as inhibition of Rac1 in vivo with EHT1864 enhances thymus cellularity in models of acute injury (Fig. 1F), and age (Fig. 1G).
As post-transplant T cell deficiency is associated with an increased risk of infections, relapse of malignancy, and the development of secondary malignancies, identifying molecular targets to enhance thymic recovery will aid in the development of therapeutics with imminent clinical need. These findings not only reveal a novel molecular mechanism governing tissue regeneration, but also offer a potentially superior therapeutic strategy for boosting thymic regeneration and T cell reconstitution after damage such as that caused by allo-HCT, infection or cytoreductive therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal