Introduction: Bruton tyrosine kinase (BTK) is a critical component of the B-cell receptor pathway, and is a validated target for the treatment of chronic lymphocytic leukemia (CLL). Ibrutinib is a first-generation, covalent, small molecule BTK inhibitor approved for the treatment of CLL. We present a preliminary analysis of treatment patterns and adverse events (AEs) in patients with CLL treated with ibrutinib in a real-world setting.
Methods: A retrospective chart review is being conducted among patients diagnosed with CLL and treated with ibrutinib in oncology centers throughout the UK; the target sample size for the study is 250 patients. Patients are eligible if they initiated ibrutinib after diagnosis of CLL, between January 2017 and June 2018, with at least 12 months of follow-up data available, with the exception that patients who died less than 12 months after ibrutinib initiation remained eligible. Hematology/oncology physicians reviewed medical records and completed web-based data collection forms. Baseline medical history information and data on treatment characteristics and AEs were collected. By June 2019, a total of 151 medical records (60% of the target sample size) had been abstracted. All analyses were descriptive in nature and were performed in SAS v9.4 or later (Cary, NC, USA).
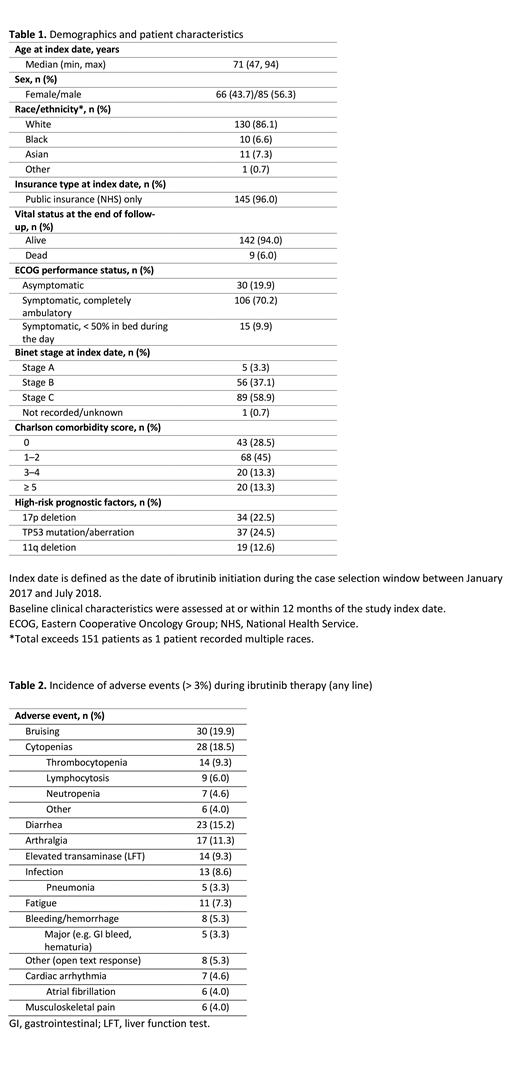
Results: Twenty-two physicians from specialist cancer centers or tertiary referral treatment centers (45.5%), teaching hospitals (31.8%) and non-teaching hospitals (22.7%) submitted data on ibrutinib-treated patients. The median follow-up for this interim sample of 151 patients was 16.1 months (range: 2.8-27.5 months) from ibrutinib initiation (index date) and 61.7 months (range: 11.6-264.1 months) from initial CLL diagnosis (Table 1). Median age was 71 years, 56% were male, 22.5% of patients had del(17p) mutation and 24.5% had TP53 mutations/aberrations. Of the 151 patients, 24.5% (n=37) initiated ibrutinib as first-line therapy while 75.5% (n=114) initiated ibrutinib as second- or later-line treatment. Median time to initiation of ibrutinib was 3.8 months (range: 0.3-123.7 months) for first-line therapy after initial CLL diagnosis and 22.3 months (range: 0.2-242.2 months) for second-line therapy after end of first-line therapy. Other therapies that patients received besides ibrutinib included the combination of fludarabine, cyclophosphamide, and rituximab (first-line, 22.5%; second-line, 1.7%), bendamustine plus rituximab (first-line, 19.9%; second-line, 15.5%), and chlorambucil plus rituximab (first-line, 10.6%; second-line, 1.7%). The most common AEs observed during ibrutinib therapy were bruising (19.9%), cytopenias (18.5%), diarrhea (15.2%), and arthralgia (11.3%) (Table 2).
Conclusion: This preliminary analysis describes patient characteristics and treatment patterns in ibrutinib-treated patients in the UK. We found that the majority of ibrutinib use was in the second-line or later, reflecting the current UK public reimbursement situation. AEs such as bruising and cytopenias were commonly reported in patients treated with ibrutinib, and future analyses from this study will determine how these AEs and others affect dosing, treatment discontinuation and healthcare resource utilization.
Xie:AstraZeneca: Employment. Yong:AstraZeneca: Employment, Equity Ownership. Waweru:AstraZeneca: Employment, Equity Ownership. Sorof:Acerta Pharma: Employment. Goyal:RTI Health Solutions: Employment. Davis:RTI Health Solutions: Employment. Follows:Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau. Hillmen:Apellis: Research Funding; Gilead: Research Funding; Roche: Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal