Introduction
ATIR101 is a donor-derived, T-cell-enriched leukocyte preparation depleted ex vivo of host-alloreactive T cells. When administered as a single, adjunctive infusion after T-cell-depleted haploidentical hematopoietic stem cell transplantation (haplo HSCT), ATIR101 may reduce serious complications resulting from delayed immune reconstitution such as infections and relapse. In a Phase II study, patients with hematologic malignancies who received ATIR101 had an overall survival (OS) of 61% and no Grade III/IV acute graft-versus-host disease (GVHD) at 1 year post HSCT (Roy DC et al. ASH 2016). Similarly, an OS rate of 55% was reported in a second Phase II study (Olavarria E et al. EHA 2018). We report multivariable and subgroup analyses of a pooled dataset from these two Phase II studies of ATIR101, compared with a dataset of control patients meeting similar inclusion/exclusion criteria who underwent T-cell-depleted haplo HSCT without ATIR101, to assess the impact of potential prognostic factors on survival outcomes.
Methods
After showing comparability allowing the pooling of data, 1-year outcomes from two Phase II studies (CR-AIR-007, NCT01794299; CR-AIR-008, NCT02500550; data cut-off June 1, 2018) including 32 adult patients who received ATIR101 (2.0 × 106 cells/kg) were compared with a historic control of 35 patients treated with a T-cell-depleted haplo HSCT without ATIR101 in centers participating in ATIR101 trials (CR-AIR-006, NCT02188290). Patients with a hematologic malignancy (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], or myelodysplastic syndromes [MDS]) underwent myeloablative or reduced-intensity conditioning with or without total body irradiation (TBI) followed by a CD34+-selected stem cell graft from a haploidentical family donor. ATIR101, prepared from the same donor, was given approximately 28 days post HSCT to patients in the ATIR101 group without prophylactic immunosuppression post HSCT. Post hoc multivariable Cox regressions for non-relapse mortality (NRM) and OS were performed on the pooled ATIR101 and control datasets adjusting for treatment, diagnosis, conditioning regimen, remission status, European Society for Blood and Marrow Transplantation risk score, and total number of viable CD34+ (× 106 cells/kg) given to patients. Variables recognized as clinically important were forced into the model regardless of whether they were statistically significant.
Results
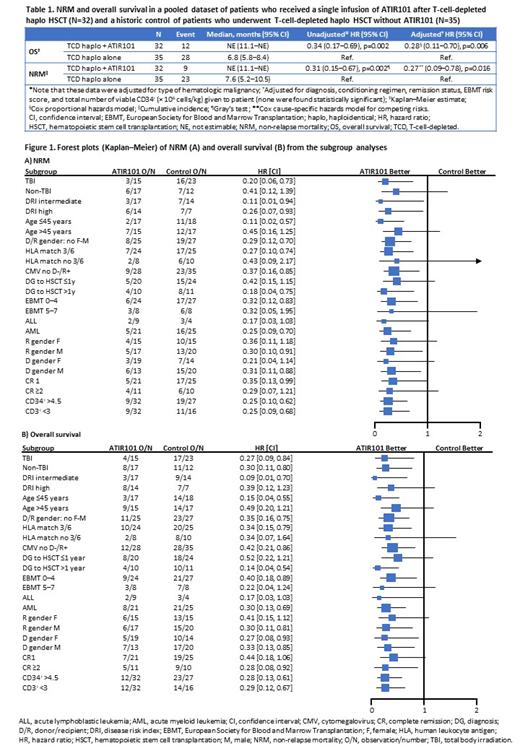
Overall, patient characteristics at baseline were balanced across groups (ATIR101 vs historic control): median age at HSCT (range) was 43 years (31-51) vs 43 years (28-54); patients had AML (66% vs 71%), ALL (28% vs 11%), or MDS (6% vs 17%); patients underwent myeloablative conditioning (100% vs 94%) with TBI (47% vs 66%) and were in complete remission (100% vs 83%) prior to transplantation. The incidence of Grade III/IV acute GVHD was similar in the ATIR101 and control groups (6%); however, only patients in the control group experienced severe chronic GVHD (2/35). Compared with the control group, the ATIR101 group showed a lower cumulative incidence of NRM (28% vs 66%; hazard ratio [HR]: 0.31; 95% confidence interval [95% CI]: 0.15-0.67; p=0.002) and a higher OS rate (63% vs 20%; HR: 0.34; 95% CI: 0.17-0.69; p=0.002). Adjusting for potential prognostic factors, the benefit of ATIR101 was confirmed in a multivariable analysis for both NRM (HR: 0.27; 95% CI: 0.09-0.78; p=0.016) and OS (HR: 0.28; 95% CI: 0.11-0.70; p=0.006; Table). The addition of ATIR101 treatment following T-cell-depleted haplo HSCT improved survival outcomes compared with T-cell-depleted haplo HSCT alone, irrespective of patient baseline characteristics, as shown in the subgroup analyses (Figure).
Conclusions
A single infusion of ATIR101 after a T-cell-depleted haplo HSCT in patients with hematologic malignancies appears to improve survival outcomes compared with a T-cell-depleted haplo HSCT alone. After adjusting for potential prognostic risk factors, the multivariable analysis appears to support the clinical benefit of ATIR101 on NRM and OS at 1 year post HSCT. A large, global, randomized Phase 3 study is currently enrolling to further examine the efficacy and safety of T-cell-depleted haplo HSCT + ATIR101 compared with T-cell-replete haplo HSCT with post-transplant cyclophosphamide (HATCY; NCT02999854).
Roy:Hopital Maisonneuve-Rosemont: Patents & Royalties: Author on patent; University of Montreal: Patents & Royalties: Author on patent; Kiadis Pharma: Other: Travel support. Walker:Kiadis Pharma: Other: Grant funding via institution (as a principal investigator). Maertens:Astellas Pharma: Other: Personal fees and non-financial support; Cidara: Other: Personal fees and non-financial support; F2G: Other: Personal fees and non-financial support; Gilead Sciences: Other: Grants, personal fees and non-financial support; Merck: Other: Personal fees and non-financial support; Pfizer: Other: Grant and personal fees; Amplyx: Other: Personal fees and non-financial support. Olavarria:Kiadis Pharma: Consultancy, Honoraria. Selleslag:Novartis: Consultancy, Honoraria, Speakers Bureau; Celyad: Other: Clinical trial research (no honoraria recieved). Wagner:Pfizer: Other: Advisory board; Novartis: Other: Advisory board; MEDAC: Other: Travel support; MSD: Other: Advisory board. Bönig:Kiadis Pharma: Other: Contract manufacturing of ATIR101; Celgene, Novartis, Sandoz Hexal: Consultancy; Sandoz Hexal, Uniqure: Research Funding; Miletenyi: Speakers Bureau. Bos:Celgene: Research Funding; Kiadis Pharma: Other: Shareholder (of Cytosen, acquired by Kiadis). Mielke:ISCT: Other: Travel support; Jazz Pharma: Honoraria, Other: Travel support, Speakers Bureau; DGHO: Other: Travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal