
Introduction
Primary plasma cell leukemia (pPCL) is a rare and aggressive plasma cell proliferation with a very poor prognosis. The prevalence of poor-risk genetic lesions is higher compared with newly diagnosed multiple myeloma. pPCL requires urgent control of clinical manifestations to prevent early death because of irreversible disease complications. The aim of the EMN12/ HOVON129 study is to improve the outcome of younger and elderly patients with pPCL by incorporating carfilzomib and lenalidomide in induction, consolidation, and maintenance. This trial was registered at www.trialregister.nl as NTR5350.
Methods
In this ongoing non-randomized, phase 2, multicenter study, patients with previously untreated pPCL receive induction therapy with carfilzomib-lenalidomide-dexamethasone (KRd; 28-day cycles with carfilzomib 20/36 mg/m2 on days 1,2,8,9,15,16; lenalidomide 25 mg days 1-21; dexamethasone 20 mg on days 1,2,8,9,15,16,22,23). In patients ≤65 years, 4 cycles of KRd induction is followed by tandem autologous stem cell transplantation (SCT), KRd consolidation, and then maintenance with carfilzomib (27 mg/m2 on days 1,2,15,16) and lenalidomide (10 mg on days 1-21/28 days) until progression. Patients who are eligible for allogeneic SCT, may also receive autologous-allogeneic tandem transplantation. Patients ≥66 years receive 8 cycles of KRd followed by carfilzomib+lenalidomide maintenance. Supportive care consists of antibacterial-, herpes zoster-, and thrombosis-prophylaxis.
Inclusion criteria are newly diagnosed pPCL (>2x109/L circulating monoclonal plasma cells or plasmacytosis >20% of the differential white cell count) and WHO-performance status 0-3. Main exclusion criteria are severe cardiac or pulmonary dysfunction; and creatinine clearance of <15 ml/min. There are no restrictions based on blood counts.
Here we report the results from the first planned interim analysis for patients aged ≤65 years. The objective of this analysis was to describe overall response rate (ORR) and toxicity of induction therapy for the first 15 registered patients aged ≤65 years.
Results
From October 2015 till May 2019, we enrolled 33 patients with pPCL, 21 aged ≤65 years and 12 aged ≥66 years. Among the first 15 patients aged ≤65 years, 47% were males; median age was 62 years (range 31-65); 67% had bone disease; and WHO performance status was 2 in 20% and 3 in 27% of patients. Patients had a high tumor burden with a median plasma cell percentage in BM biopsy of 90%. The median peripheral blood plasma cell count was 5.2x109/L (range 2.0-27.4); median platelet count was 121x109/L (range 27-289); and median GFR was 53 ml/min (range 15-81 ml/min). Patients had high-risk disease as evidenced by ISS stage 3 in 67%; elevated LDH in 53%; and high frequency of high-risk genetic lesions (del(17p) in 58%, t(4;14) in 8%, t(14;16) in 8%, del(1p) in 50%, ampl(1q) in 58%). In addition, 20% of patients had extramedullary disease.
Among the first 15 registered patients, 14 received the planned 4 cycles of induction treatment, while one patient went off protocol after cycle 3 due to disease progression. After 4 induction cycles, (stringent) CR was achieved by 5 (33%), at least VGPR by 12 (80%), and ≥PR by 14 patients (93%).
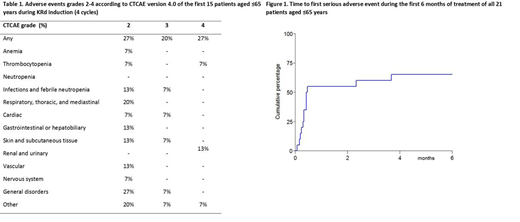
Table 1 shows adverse events that occurred throughout induction treatment. Adverse events mainly occurred during the first cycle of KRd, and decreased thereafter. Hematological toxicity was limited. One patient experienced a grade 3 infection. Cardiac events occurred in 2 patients: 1 grade 3 myocardial infarction, and 1 grade 2 heart failure. No patients discontinued treatment because of toxicity. Mortality during induction was 0%.
In all 21 patients aged ≤65 years, 14 (67%) experienced one or more serious adverse events (SAEs), mainly hospitalizations. The majority of these SAEs occurred during the first KRd cycle (Figure 1).
Conclusions
KRd induced deep hematologic responses after 4 cycles of therapy (≥VGPR in 80% and ≥CR in 33%) without early death. Toxicity consisted of 20% grade 3 and 27% grade 4 events, occurred mainly during the first cycle of induction, and was manageable with appropriate interventions. In conclusion, KRd provides efficient and rapid disease control, which is essential to prevent early mortality because of irreversible disease complications and to improve survival of patients with this aggressive plasma cell proliferative disorder.
Van De Donk:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees. Schjesvold:Novartis: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; SkyliteDX: Honoraria; MSD: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Broyl:Celgene, amgen, Janssen,Takeda: Honoraria. Minnema:Jansen Cilag: Honoraria; Servier: Honoraria; Gilead: Honoraria; Amgen: Honoraria; Celgene Corporation: Honoraria, Research Funding. Offidani:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Caers:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Zweegman:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Sonneveld:Janssen: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; SkylineDx: Research Funding; BMS: Honoraria; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Hajek:Amgen: Honoraria, Other: Consultant or advisory relationship, Research Funding; Celgene: Honoraria, Other: Consultant or advisory relationship, Research Funding; AbbVie: Other: Consultant or advisory relationship; Bristol-Myers Squibb: Honoraria, Other: Consultant or advisory relationship, Research Funding; Novartis: Other: Consultant or advisory relationship, Research Funding; PharmaMar: Honoraria, Other: Consultant or advisory relationship; Takeda: Honoraria, Other: Consultant or advisory relationship, Research Funding; Janssen: Honoraria, Other: Consultant or advisory relationship, Research Funding. Gay:AbbVie: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Vangsted:Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Jansen: Honoraria. Musto:Celgene: Honoraria; AMGEN: Honoraria.
KRd is used for primary plasma cell leukemia
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal