Background
Hemophagocytic lymphohistiocytosis (HLH) is an inflammatory condition caused by uncontrolled proliferation of activated lymphocytes and macrophages secreting an excess of inflammatory cytokines. When untreated, primary HLH is invariably fatal. Treatment requires the achievement of remission of HLH prior to allogeneic hematopoietic stem cell transplantation. Despite significant treatment progress, pre-HSCT mortality remains a challenge. In the Etoposide-based HLH-94 and HLH-2004 studies pre-HSCT mortality was 27% and 19%, respectively.
A better understanding of the pathophysiology of primary HLH has opened new avenues for targeted immunotherapy. Based on our previous observation concerning the use of Antithymoglobulin in HLH, we propose a new therapeutic strategy with Alemtuzumab in association with steroids and cyclosporine A (CSA) as first line treatment in primary HLH. In contrast to ATG, Alemtuzumab does not activate T lymphocytes while killing them. Therefore, we expect a better tolerance and efficacy of Alemtuzumab. This may have a positive impact not only on survival until HSCT, but also on overall survival and quality of life, especially with regard to long-term neurological sequelae.
Methods
24 consecutive treatment naïve patients with genetically confirmed primary HLH had received first line Alemtuzumab in association to steroids and CSA from 01/2009 to 06/2015 in the Unit for Pediatric Immunology in Necker Hospital Paris, as well as two additional patients in 10/2016 and 10/2018 respectively, who could not be included in the prospective trial.
From 06/2015 to 06/2019, 29 patients have been enrolled in a multicenter, open, phase I/II, non-comparative, non randomized study (NCT02472054). Patients with lymphohistiocytic activation syndrome who had not received any specific treatment prior to enrollment except steroids and CSA were included. Treatment consisted in intravenous administration of Alemtuzumab in association to Methylprednisolone and CSA.
The primary outcome measures is the number of surviving patients until HSCT, secondary outcome measures the number of complete remissions following treatment at Day (D)14, D21, D28. To assess the efficacy of the Alemtuzumab, the time of delay between the first administration of Alemtuzumab and complete remission will be determined. Alemtuzumab Pharmacokinetics will be done. All adverse events are reported.
Results
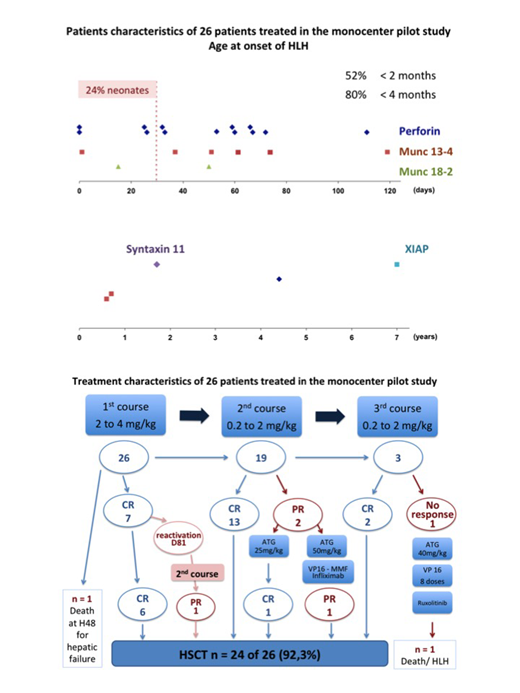
Retrospective analysis of 26 patients (pilot study): The median age of patients was 1.9 months (birth - 7 years), 6 patients were neonates. When Alemtuzumab was started, out of 26 patients 12 (46.1%) required intensive care, 8 (30.7%) mechanical ventilation, 13 (50%) had neurological involvement, 9 (34.6%) hepatocellular insufficiency. One 2-month-old Munc13-4 patient died at H+48 after two administrations of Alemtuzumab (total dose 1.5mg/kg) for hepatic failure and acute renal failure. A second patient with Perforin deficiency did not respond neither to three courses of Alemtuzumab (cumulative dose 6.5mg/kg) nor repeated Etoposid, 40mg/kg ATG, or Ruxulotinib. He died at D+65. The 24 remaining patients survived until HSCT (survival 92.3%). As shown in the figure, two patients required additional treatment. Overall 22 patients achieved CR, 2 PR at the time of HSCT.
The prospective study enrolled 29 patients from 06/2015 to 06/2019. Median age at onset of HLH was 0.5 years (range 0.02 to 17.2 years), one patient withdrawed consent. 12 patients received one course, 13 two, 2 three and one patient 4 courses of Alemtuzumab. 24 patients with a genetic confirmed HLH predisposition reached the primary endpoint with 22 surviving until HSCT (91,6%). One patient is still awaiting HSCT. The three remaining patients are one CA-EBV patient and a newborn with secondary HLH due to fulminant HSV hepatitis, who both died, as well as a patient with predominant neurological HLH without genetic diagnosis who is in sustained remission without any specific treatment. Detailed results from the completed study will be presented.
Conclusions
This is the first report on Alemtuzumab as first line approach in the treatment of primary HLH. Our results in more than 50 pediatric patients treated in a pilot study and prospective trial indicate that Alemtuzumab allows controlling HLH activity with a favorable safety and tolerability profile in a very fragile population. 92.3% and 91.6% of patients respectively survived to HSCT.
No relevant conflicts of interest to declare.
Alemtuzumab (Campath) has been used in a prospective trial to evaluate its efficacy as first line treatment in Familial Lymphohistiocytosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal