Sickle cell disease (SCD) is an inherited blood disorder in which red blood cells are sickle-shaped as a result of an amino acid alteration from glutamate to valine at the sixth position of the β-globin chain. Hematopoietic stem cell transplant (HSCT) is a curative option for SCD, with both HLA-matched and haploidentical transplant being viable approaches. Stable mixed chimerism and tolerance induction are sufficient to reverse the sickle phenotype. Graft rejection and graft versus host disease may occur following nonmyeloablative haploidentical peripheral blood stem cell transplantation (PBSCT).
Various regulatory cytokines have been identified as biomarkers of engraftment in graft transplantation. Previously, we observed that TGF-β, interleukin (IL)-7, and IL-10 cytokines were elevated in engrafted SCD patients as compared to those who rejected their grafts following haploidentical PBSCT. Now, we aim to identify the immune cell populations responsible for producing the above cytokines in both engrafted and rejected patients.
The plasma samples of 21 SCD patients who underwent nonmyeloablative haploidentical PBSCT at the National Institutes of Health between March 2010 and September 2015 were evaluated previously by multiplexed magnetic bead enzyme linked immune sorbent assay. Here, we analyzed various immune cells which produce the above regulatory cytokines through multi-color flow cytometry in 8 out of the 21 patients (four engrafted and four rejected). We evaluated the cytokine producing capabilities via intracellular cytokine detection among T regulatory (Treg, CD3+CD4+CD25+Foxp3+) cells, Type 1 regulatory (Tr1, CD3+CD4+Foxp3-CD45RA-CD49+LAG3+) T cells, T helper 1 (Th1, CD3+CD4+Foxp3-CD45RO+CXCR3+) cells, T helper 17 (Th17, CD3+CD4+Foxp3-CD45RO+CCR6+) cells, natural killer (NK, CD3-CD56+NKG2D+) cells, B regulatory (Breg, CD3-CD56-CD19+CD24hiCD38hi)cells, polymorphonuclear-myeloid derived suppressor cells (PMN-MDSCs, CD45+CD14-CD15+CD11b+), monocytic-MDSCs (CD45+CD14+CD15-HLADRlow), early-MDSCs (CD45+Lin-HLADR-CD11b+CD33+), myeloid dendritic cells (mDCs, CD45+CD3-CD56-HLADR+CD11c+CD123-), and plasmacytoid dendritic cells (pDCs, CD45+CD3-CD56-HLADR+CD11c-CD123+). Cytokine producing cell frequencies were measured at five timepoints: baseline (day 0) and days 30, 60, 100, and 180 post-transplant (PT). Percentages of intracellular cytokine and cell population frequencies were assessed through flow cytometry analysis and statistical significance was determined by Student's t-test.
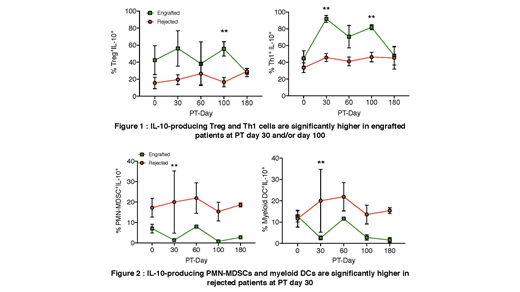
We noted IL-10-producing Tregs were significantly elevated in engrafted patients at PT day 100 (p< 0.01, see Figure 1). Further, IL-10-producing Th1 cells were significantly higher in engrafted patients on PT day 30 (p< 0.01) and day 100 (p< 0.01). However, rejected patients had significantly higher amounts of IL-10-producing PMN-MDSCs (p< 0.01), and myeloid DCs (p< 0.01, see Figure 2), but their levels were lower than IL10-producing Tregs and Th1 cells of engrafted patients. There were no significantly different alterations of IL-10-producing cell frequencies among other cell populations (Tr1, Th17, NK, Breg, early-MDSCs, monocytic-MDSCs, and pDCs). In addition, there were no significant variations in TGF-β- and IL-7-producing cell frequencies in any of the immune cell populations tested.
Overall, the engrafted patients showed higher IL-10-producing Tregs and Th1 cells at PT day 30 and/or day 100, which suggest that the immunosuppressive cytokine IL-10 may play an important role in engraftment. Further analyses with a larger sample size are indicated to evaluate whether IL-10 producing immune regulatory cells may serve as a biomarker to predict transplant outcome. Taken together, continuous regulatory cytokine analysis at serial time points pre- and post-transplant may aid in establishing mechanisms of successful engraftment and tolerance induction.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal