Introduction
Immune thrombocytopenia (ITP) is characterized by platelet (plt) destruction and impaired plt production related to multiple humoral and cell-mediated processes. The heterogeneity of ITP pathogenesis is highlighted by clinical experience that shows up to 20% of patients with ITP have an inadequate response to current therapy and remain at risk of life-threatening bleeds and substantially impaired quality of life. Evidence of activation of the classical complement pathway (CP) by plt autoantibodies has long been recognized in ITP. In vitro studies showed sera from approximately 50% of patients with ITP activated the CP and/or fixed complement on plt surfaces. Inhibition of the CP in vitro with TNT003, a monoclonal C1s antibody, decreases deposition of C3b and C5b-9 on plts when exposed to ITP patient sera. Sutimlimab (formerly BIVV009) is a humanized, monoclonal antibody that selectively inhibits C1s, the proximal mediator of the CP, and has shown clinical activity in other CP-mediated disorders, such as cold agglutinin disease. We hypothesized that a primary mechanism of thrombocytopenia in a subset of patients with ITP is CP dependent, and that CP inhibition with sutimlimab should improve thrombocytopenia.
Methods
Adult patients with chronic (>1 year of duration), severe ITP with inadequate response to ≥2 prior therapies (plt count [ct] ≤30 × 109/L at screening) were enrolled in an open-label Phase 1 trial. Patients could receive concomitant ITP medication if on a stable dose for the prior month and were unable to sustain plt cts ≥30 × 109/L in the absence of bleeds. Patients received sutimlimab on Day 0, 7, and then biweekly for up to 21 weeks (Part A) followed by a scheduled washout to evaluate relapse and re-treatment response in a re-treatment/continuation arm (Part B) for an additional year.
Results
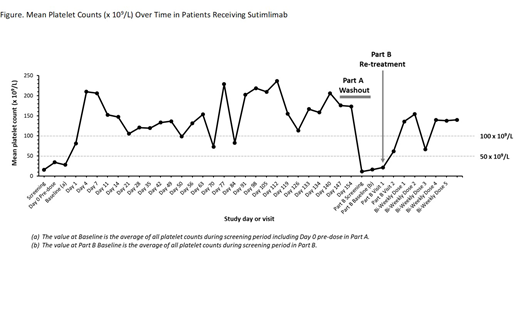
All results are presented as of a data cutoff of 14 December 2018. Seven patients received at least 1 dose of sutimlimab. At screening for Part A, mean (range) age was 44.9 (28-65) years and 85.7% (n=6) of patients were female. The mean (range) baseline plt ct for Part A was 27.9 × 109/L (8-57). The mean (range) plt ct 24 hours post-initial dose of sutimlimab was 81.3 × 109/L (1-209). The mean (range) plt ct on Day 7 was 206.3 × 109/L (25-435). Mean plt ct was maintained at >50 × 109/L throughout Part A. Response defined as a plt ct >50 × 109/L measured on 2 separate occasions more than 7 days apart was achieved by 57% (n=4) of patients by Day 14 (Figure). One additional patient achieved a stable plt ct 20-50 × 109/L. The mean (range) C4 level increased from 27.6 (15-45) mg/dL at baseline to 37.3 (26-50) mg/dL at Day 7 after the initial sutimlimab treatment. Four patients completed the protocol washout/re-treatment at the end of Part A and entered Part B. In all of these patients, sutimlimab washout resulted in reoccurrence of thrombocytopenia. Mean (range) time in washout was 4.1 (3-7) weeks. These 4 patients were re-treated in Part B. The mean (range) baseline plt ct at re-treatment was 16.0 × 109/L (5-26). Re-treatment efficacy was observed in patients in Part B. Safety data is available from Part A as of the 14 December 2018 data cutoff. Six patients experienced a total of 30 treatment-emergent adverse events. No patients discontinued Part A early due to an adverse event. Two patients experienced a total of 3 treatment-emergent serious adverse events (SAE), of which 1 SAE of migraine was assessed as possibly related to sutimlimab. There were no thrombotic events. The benefit-risk remained positive for continued investigational use of sutimlimab in ITP.
Conclusions
In a preliminary analysis of interim data, sutimlimab resulted in a rapid (<24 hours), sustained increase in plt ct in patients with chronic ITP who had inadequate responses to ≥2 currently available therapies at trial entry. Washout kinetics demonstrated that thrombocytopenia reoccurs when sutimlimab is discontinued, and that thrombocytopenia resolution occurs upon re-treatment. This is the first clinical evidence that the CP plays a role in thrombocytopenia in a subset of patients with ITP. These responses suggest at least 1 additional pathophysiologic explanation for the clinical heterogeneity of ITP, and provide a strong rationale for continued evaluation of CP inhibition in ITP treatment.
Broome:Sanofi Genzyme: Honoraria, Research Funding; Cellphire: Research Funding; Rigel: Research Funding; Incyte: Research Funding; Alexion: Honoraria, Research Funding. Röth:Bioverativ: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kuter:Daiichi Sankyo: Consultancy, Honoraria; Caremark: Consultancy, Honoraria; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Zafgen: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Platelet Disorder Support Association: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Platelet Disorder Support Association: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Dova: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Dova: Consultancy, Honoraria; Shinogi: Consultancy, Honoraria; Zafgen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Principia: Consultancy, Honoraria, Research Funding; Merck Sharp Dohme: Consultancy, Honoraria; Merck Sharp Dohme: Consultancy, Honoraria; Protalix: Consultancy, Honoraria; Shinogi: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Caremark: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Shire: Consultancy, Honoraria; UCB: Consultancy, Honoraria; UCB: Consultancy, Honoraria; Principia: Consultancy, Honoraria, Research Funding; Momenta: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Kezar: Research Funding; Kezar: Research Funding; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria, Research Funding; Rigel: Consultancy, Honoraria, Research Funding; Rigel: Consultancy, Honoraria, Research Funding; Protalix: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Research Funding; Alnylam: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Alnylam: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria. Scully:Shire/Takeda: Consultancy; Shire: Research Funding; Ablynx/Sanofi: Consultancy; Novartis: Consultancy; Alexion: Consultancy. Smith:Alnylam: Honoraria; Bioverativ: Honoraria. Wang:Sanofi: Employment. Reuter:Pediatric Infectious Disease Society: Other: Social Media Committee, Vaccine Advocacy Committee; Sanofi: Employment. Hobbs:Sanofi-Genzyme: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal