
Introduction
Rozanolixizumab targets the human neonatal Fc receptor (FcRn). By blocking IgG recycling, this first-in-class subcutaneously (SC) infused monoclonal antibody aims to improve the course of immunoglobulin G (IgG)-mediated autoimmune diseases by reducing pathogenic autoantibody levels. We report the completed Phase II, open-label study of rozanolixizumab in patients (pts) with primary immune thrombocytopenia (ITP; NCT02718716).
Methods
Eligibility: ≥18 yrs old; primary ITP ≥3 months (persistent/chronic); platelet count <30x109/L (screening) and <35x109/L (baseline). Pts received single (15 or 20 mg/kg) or multiple (5 x 4 mg/kg, 3 x 7 mg/kg, 2 x 10 mg/kg weekly) doses of rozanolixizumab; total dose in each group ranged from 15-21 mg/kg. Treatment was infused SC (30-90 mins). An 8-week observation period followed the last study treatment. Primary objective: safety and tolerability of rozanolixizumab (occurrence of adverse events [AEs]); secondary objective: clinical efficacy (changes in platelet count) and pharmacodynamic effect (changes in IgG levels).
Results
Of the 66 pts enrolled, 65 (98.5%) completed the study (one discontinued due to lack of efficacy, 4 mg/kg multiple-dose cohort). All pts were included in the safety set (SS) and full analysis set (FAS) while 64 were included in the per protocol set (PPS) and pharmacodynamic set (PD)-PPS. Two pts were excluded due to protocol deviations: procedural non-compliance and/or prohibited medication use.
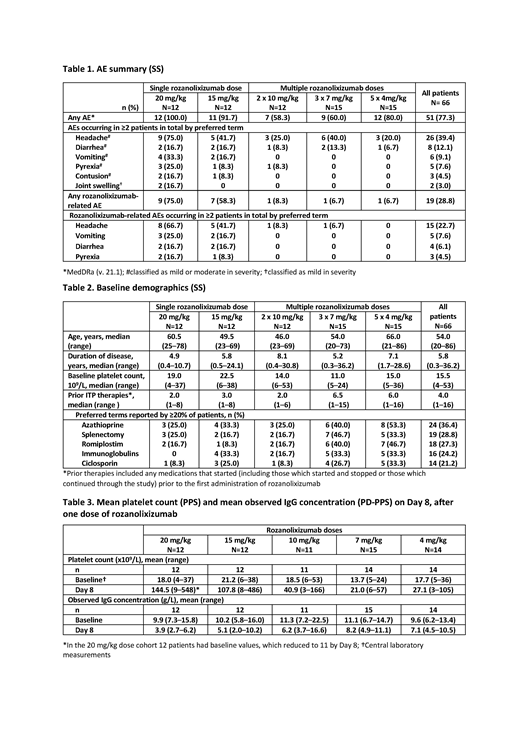
Overall, 51/66 (77.3%) pts reported ≥1 AE, mostly mild-to-moderate headaches (26/66 [39.4%]; Table 1). None of 4 serious AEs was treatment-related: genital tract bleeding (4 mg/kg group), thrombocytopenia (10 mg/kg), thrombocytopenia and platelet count decrease (both 15 mg/kg). One pt in each multiple-dose group and 7/12 and 9/12 pts (15 and 20 mg/kg, respectively), reported AEs (mostly headaches) deemed treatment-related (Table 1). No serious infections were seen.
Baseline disease characteristics suggested a difficult to treat population, with a median of 5.8 years ITP duration, 4 prior ITP therapies and approx. 30% of pts having previously received thrombopoetin receptor agonists (Table 2). More pts receiving a single (15 and 20 mg/kg) rozanolixizumab SC infusion achieved platelet count ≥50x109/L (8/12 [66.7%] and 6/11 [54.5%; one pt excluded] in the 15 and 20 mg/kg groups, respectively) compared with those receiving multiple SC infusions: 5/14 (35.7%), 5/14 (35.7%; one pt excluded) and 5/11 (45.5%) in the 4, 7 and 10 mg/kg groups, respectively. In particular, median time to platelet count ≥50x109/L was considerably shorter in pts receiving a single dose of rozanolixizumab (7 and 5 days in the 15 and 20 mg/kg groups, respectively) compared with multiple SC infusions (14, 14 and 8 days in the 4, 7 and 10 mg/kg groups, respectively). For early responses by Day 8, a platelet count of ≥50x109/L was achieved by 7/12 (58.3%) and 6/11 (54.5%) pts in the 15 and 20 mg/kg dose groups, respectively (13/23 pts receiving a single infusion), compared with 1/14 (7.1%), 2/14 (14.3%) and 3/11 (27.3%) pts in the 4, 7 and 10 mg/kg groups, respectively (6/39 pts receiving multiple infusions).
Dose-dependent increases in platelet count were observed with peak median counts >100x109/L in the 15 and 20 mg/kg groups (Table 3).
Dose-dependent decreases in mean serum IgG concentrations were observed by Day 8 (Table 3). The 20 mg/kg single-dose group achieved their nadir on Day 8 (mean IgG concentration: 3.9 g/L, reduction of 5.9 g/L (60.0%) from baseline), while the 5 x 4 mg/kg group achieved their nadir on Day 29 (mean IgG concentration: 5.6 g/L, reduction of 4.0 g/L (43.6%) from baseline). Exploratory analyses, using data at baseline and Day 8, suggested that the magnitude of serum IgG decrease was associated with the corresponding platelet increase.
Conclusion
Rozanolixizumab was well tolerated across all dose groups (4-20 mg/kg) with mild-to-moderate headaches seen at higher doses; no patient discontinued the study due to side effects. While improvements in platelet count and decreases in IgG levels were seen at all doses of rozanolixizumab, the single SC infusions (15 and 20 mg/kg) achieved these efficacy endpoints both sooner (by Day 8), more frequently and at numerically greater levels than multiple doses. These safety, tolerability, and efficacy data support Phase III development of rozanolixizumab in patients with primary ITP.
Robak:Takeda: Consultancy, Research Funding; UCB: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel grant, Research Funding; Amgen: Consultancy, Other: Travel grant; Roche: Consultancy, Other: Travel grant, Research Funding; Abbvie: Consultancy, Honoraria, Other: Travel grant, Research Funding; Gilead: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Acerta: Research Funding; Morphosys AG: Research Funding. Jarque:Abbie: Consultancy, Speakers Bureau; Alexion: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; CellTrion: Consultancy; Gilead: Consultancy, Speakers Bureau; Grifols: Consultancy; Janssen: Consultancy, Speakers Bureau; MSD: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Servier: Speakers Bureau; Shionogi: Consultancy, Speakers Bureau; Shire: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau. Musteata:Arensia EM: Other: Principal Investigator; Institute of Oncology: Employment. Cooper:Principia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kiessling:UCB Biosciences: Employment, Equity Ownership. Massow:UCB Biosciences GmbH: Employment. Woltering:UCB: Employment. Snipes:UCB Biosciences Inc: Employment. Ke:UCB Pharma: Employment, Equity Ownership. Langdon:AstraZeneca: Consultancy; Conatus: Consultancy; Pfizer: Consultancy; UCB Pharma: Consultancy; Ziarco: Consultancy; MMV: Consultancy; Sigmoid Pharma: Consultancy; Mylan: Consultancy; Creablis: Consultancy. Haier:UCB Pharma Sprl: Employment, Equity Ownership. Bussel:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Momenta Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Dova Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; 3S Bio: Speakers Bureau; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Tranquil: Honoraria, Membership on an entity's Board of Directors or advisory committees; Physician Education Resource: Speakers Bureau; Kezar Life Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; RallyBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; argenx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Jolles:UCB Pharma: Consultancy, Other: Drug Safety Committee; CSL Behring: Consultancy, Honoraria, Research Funding, Speakers Bureau; Shire/Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Octapharma: Consultancy, Honoraria, Research Funding, Speakers Bureau; LFB: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharming: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal