Genomic studies of myeloid malignancies (MM), including acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN) and myelodysplasia (MDS), identified mutations with different allele frequencies. Recent studies of clonal hematopoiesis (CH) discovered a subset of MM disease alleles, while other alleles are only observed in overt MM. These observations suggest an important pathogenetic role for the chronology of mutational acquisition. Although bulk sequencing informs prognostication, it cannot distinguish which mutations occur in the same clone and cannot offer definitive evidence of mutational order. Delineation of clonal architecture at the single cell level is key to understanding how the sequential/parallel acquisition of somatic mutations contributes to myeloid transformation.
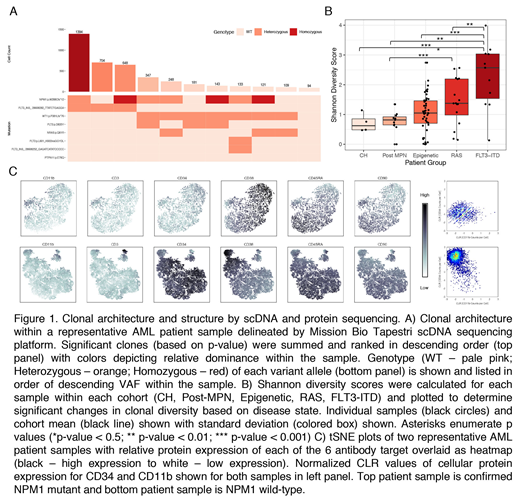
In order to elucidate the clonal structure of MM, we designed a custom single cell 109 amplicon panel of the most frequently mutated amplicons in 50 MM genes using the Mission Bio Tapestri v2 platform. Viable cells were sorted from 90 samples from 78 patients with CH, AML, and MPN/post-MPN AML followed by single cell amplification/sequencing. Mutation calls were filtered based on read depth, quality, and alleles genotyped per cell. We reconstructed a random distribution of clones by permuting genotype calls across cells and generated empirical p values for each clone. To identify dominant clones, we used a Poisson test to determine clones were significantly enriched compared to the mean clone size. Clones with significant p-values (p <0.05) were used to generate plots of clonal architecture of each sample (Figure 1A).
Despite significant clonal complexity, the majority of MM patients (80%;72/90) present with one (51/90; 56.7%) or two (21/90; 23.3%) dominant clones. These data show there are specific genotypic combinations which lead to clonal dominance with increased fitness relative to other clones and/or suppression of minor clones by dominant clone(s). We next investigated whether specific molecularly defined AML subtypes had increased clonal complexity. FLT3-ITD mutant AML samples had a significantly greater number of clones (p < 0.002) compared to AML samples with multiple epigenetic modifier mutations. Similar findings were not observed when comparing AML samples with epigenetic mutations to RAS pathway mutant samples.
We next investigated whether specific mutations were likely to co-occur/be mutually exclusive at a single cell level. We observed evidence of oligoclonality in CH, including parallel acquisition of DNMT3A mutations and clones with multiple mutations in the absence of progression to MM. By contrast, in MM the dominant clone(s) almost always harbored multiple epigenetic modifier mutations, suggesting cooperative epigenetic remodeling in myeloid transformation. Mutations in signaling effectors (FLT3-ITD/TKD; RAS/RAS) were mutually exclusive. We observed distinct FLT3-mutant clones in FLT3-mutant AML patients and parallel acquisition of different RAS pathway mutations. We used this data to develop clonal architecture trees in each patient, giving us a definitive picture of mutational acquisition and transformation at a single cell level. We calculated a Shannon diversity score and observed an increase in clonal complexity with disease evolution; CH samples had the lowest clonal diversity and FLT3-ITD AML patients the highest clonal diversity (Figure 1B).
We extended our findings by combining cell surface marker assessment and single cell mutational analysis. Patient samples were stained with an antibody cocktail of 6 oligo-conjugated antibodies with barcode tags prior to single cell sequencing, which allowed simultaneous acquisition of single cell immunophenotypic and genotypic data. This allows us to identify distinct populations of stem/progenitor cells with distinct clonal/mutational repertoires (Figure 1C). Additional data will be presented with this novel approach, which allows us to combine an assessment of stem/progenitor cell frequency with genetic data. This includes studies of CD34+ and CD34- AML, which show striking differences in mutational representation in different stem/progenitor compartments.
In summary, our studies of clonal architecture at a single cell level provide us novel insights into the pathogenesis of myeloid transformation and give us new insights into how clonal complexity contributes to disease progression.
Ooi:Mission Bio: Employment, Equity Ownership. Mendez:Mission Bio: Employment, Equity Ownership. Carroll:Janssen Pharmaceuticals: Consultancy; Incyte: Research Funding; Astellas Pharmaceuticals: Research Funding. Papaemmanuil:Celgene: Research Funding. Viny:Mission Bio: Other: Sponsored travel; Hematology News: Membership on an entity's Board of Directors or advisory committees. Levine:Roche: Consultancy, Research Funding; Amgen: Honoraria; Imago Biosciences: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Qiagen: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Prelude Therapeutics: Research Funding; Loxo: Membership on an entity's Board of Directors or advisory committees; Lilly: Honoraria; Gilead: Consultancy; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal