Key Points
Initial high-risk disease, disease progression, not reaching CR before transplantation are risk factors in patients with relapsed BL/B-AL.
Time-condensed continuous infusion chemoimmunotherapy followed by stem cell transplantation forms the basis for future studies.
Abstract
Children with refractory or relapsed Burkitt lymphoma (BL) or Burkitt leukemia (B-AL) have a poor chance to survive. We describe characteristics, outcome, reinduction, and transplantation approaches and evaluate risk factors among children with progression of a BL/B-AL included in Non-Hodgkin’s Lymphoma-Berlin-Frankfurt-Münster studies between 1986 and 2016. Treatment recommendation was reinduction including rituximab from the early 2000s followed by blood stem cell transplantation. The 3-year survival of the 157 children was 18.5 ± 3%. Survival significantly improved from 11 ± 3% before to 27 ± 5% after 2000 (P < .001), allowing for risk factor analyses among the latter 75 patients. Survival of 14 patients with relapse after initial therapy for low-risk disease (R1/R2) was 50 ± 13% compared with 21 ± 5% for 61 patients progressing after R3/R4 therapy (P < .02). A total of 25 of 28 patients with progression during first-line therapy, 31 of 32 with progression during reinduction, 15 of 16 not reaching a complete remission (CR) before transplantation, 9 of 10 treated with rituximab front-line, and all 13 patients not receiving rituximab during reinduction died. Forty-six patients received stem cell transplantation (20 autologous, 26 allogeneic). Survival after a regimen combining rituximab with continuous-infusion chemotherapy followed by allogeneic transplantation was 67 ± 12% compared with 18 ± 5% for all other regimen and transplantations (P = .003). Patients with relapsed BL/B-AL have a poor chance to survive after current effective front-line therapies. Progression during initial or reinduction chemotherapy and initial high-risk disease are risk factors in relapse. Time-condensed continuous-infusion reinduction followed by stem cell transplantation forms the basis for testing new drugs.
Introduction
The efficacy of current front-line therapies for childhood mature B-cell non-Hodgkin lymphoma (B-NHL) leaves few patients with disease progression during initial therapy (including refractory disease) or relapse to study reinduction and consolidation approaches.1-6 The limited reports on progressive/relapsed B-NHL are with one exception based on retrospective surveys including different B-NHL subtypes.6-14 Some analyses are restricted to patients with relapse after initial therapy, thereby excluding 30% of Burkitt lymphoma (BL) patients with progression during first-line therapy.7,10-12,14 The strategy followed in relapse in all groups was intensive reinduction followed by hematopoietic stem cell transplantation (SCT).
Survival in relapse differs considerably according to the B-NHL subtype. Patients with relapse of a BL or Burkitt leukemia (B-AL) had a significantly poorer chance to survive, only 10% to 20%, compared with patients with relapse of a diffuse large B-cell lymphoma or primary mediastinal large B-cell lymphoma.7,10,12 The limited number of patients included in the reported series and exclusion of patients with progressive disease during initial therapy until now prohibited analysis of characteristics, treatment approaches, outcome, and risk factors among children with refractory or relapsed BL and B-AL.
Among all patients with relapsed mature B-NHL high intensity of front-line therapy, short time from first diagnosis to failure, high initial LDH level, and bone marrow involvement at relapse have been described as major risk factors for survival.6,7,12 The chance to survive was almost 0 for patients who progressed during initial therapy or were refractory to reinduction chemotherapy.7,10,11 The failure rate of the reinduction chemotherapy regimen, meaning fatal disease progression before SCT, was in the range of 50% and evidently did not vary between chemotherapies in most series.7,10-12 Only patients who reached a second remission and received SCT for consolidation had a chance to survive.7,10,12 Available analyses show no advantage of allogeneic over autologous SCT for the outcome of children/adolescents with relapsed mature B-NHL.12,14,15
We analyzed characteristics, reinduction and consolidation approaches, outcome and risk factors in a population-based unselected series of 157 BL/B-AL patients with refractory disease, progression during or relapse after BFM-type front-line therapy (collectively called progression from now on), and a stable rate of progression of 8% before the general introduction of front-line rituximab.
Patients and methods
Patients
Between September 1986 and December 2016, 157 of 1979 patients (7.9%) included in studies or registries of the Non-Hodgkin’s Lymphoma-Berlin-Frankfurt-Münster (NHL-BFM) study group from Austria, the Czech Republic, Germany, and Switzerland with cytology- or histology-confirmed refractory or relapsed BL (n = 1539, 93 relapses) or B-AL (n = 440, 64 relapses) during or after first-line chemotherapy were registered to the NHL-BFM study center. The patients underwent central histology or cytology review of their initial diagnosis confirming BL or B-AL. Reference histology included immunohistology. Staging bone marrow smears and CSF-cytospins were centrally reviewed as well. Initial staging and adequate therapy were monitored by the NHL-BFM study center. All patients or their parents gave informed consent for transfer of their data to the NHL-BFM study center.
Patients with BL/B-AL after organ transplantation or those with known immunodeficiency were not included in the analysis. Patients with confirmation of a BL as second malignancy were excluded, as were relapse patients who did not receive risk-adapted front-line therapy of adequate intensity according to their risk group.
First-line therapy
The 157 patients were included in the consecutive NHL-BFM study protocols 86, 90, 95, and B-NHL-BFM 04, or registered to the NHL-BFM Registry 2012.4,5,16 All 4 protocols and standard treatment from June 2012 on consisted of risk-adapted short-pulse chemotherapy courses over a period of 2 to 5 months. The patients were stratified according to clinical stage, initial resection status, lactate dehydrogenase (LDH) level, and central nervous system (CNS) involvement into 4 risk groups (R1-R4) (definition in supplemental Table 1, available on the Blood Web site). The progression rates during/after these BFM-type protocols were similar, ∼8%.4,5,16 The treatment intensity and the drugs were comparable among the study protocols, with the exception that 8 patients were included in the rituximab phase 2 trial between 2004 and 2011.17
Relapse therapy
Since the mid-1990s, the treatment recommendation for patients with a first relapse of a mature B-NHL consisted of reinduction chemotherapy by the NHL-BFM courses CC and AA and subsequent autologous SCT after Busulfan-based conditioning (supplemental Figure 1). From April 1995, this approach became written study recommendation within protocol NHL-BFM95. Approval of the study was obtained from the ethical committees of the principal and participating investigators. The recommended relapse chemotherapy consisted of 2 to 3 5-day courses CC, AA (and BB) (supplemental Figure 1). The recommendation for high-dose therapy for patients older than 2 years of age consisted of busulfan, etoposide, and cyclophosphamide, and thiotepa-based for CNS-positive patients.
From 2001 on, rituximab was generally available, recommended and given to most patients with relapsed BL/B-AL. In addition, other reinduction chemotherapies were applied that can be grouped according to the following schemes: CC-based; rituximab, ifosfamide, carboplatin, and etoposide (R-ICE); ifosfamide, carboplatin, idarubicin/mitoxantrone, paclitaxel, and rituximab (R-ICI/ICN); rituximab, vincristine, idarubicin, ifosfamide, carboplatin, and dexamethasone (R-VICI) (supplemental Figure 1); and other regimen. Furthermore, allogeneic SCT was performed increasingly from 2001 on at the discretion of the treating physician.
Statistical analysis
Univariate analysis was conducted by the Wilcoxon test for quantitative variables and Fisher's exact test for qualitative variables. When frequencies were sufficiently large, χ2 test was used.
Survival was calculated from date of relapse to death of any cause or last follow-up.
Probabilities of survival were estimated using the Kaplan-Meier method with standard errors according to Greenwood and were compared with the log-rank test.18 Cumulative incidence functions of relapse and secondary malignancy were constructed by the method of Kalbfleisch and Prentice.19 Functions were compared with the Gray test.20
Computations were performed using SAS (Statistical Analysis System, version 9.4, SAS Institute Inc, Cary, NC).
Results
Survival according to treatment period
With a median follow-up of 6.1 years (1.6-9.6), the probability of survival after first progression for all 157 patients was 22.5 ± 3% at 1 year and 18.5 ± 3% at 3 and 5 years (Figure 1A).
Survival at 4 years of 157 patients with relapse or progression of a BL or B-AL. (A) During or after appropriate risk-adapted NHL-BFM front-line therapy between 1986 and 2016, and (B) according to the time of relapse (1986-2000 vs 2001-2016).
Survival at 4 years of 157 patients with relapse or progression of a BL or B-AL. (A) During or after appropriate risk-adapted NHL-BFM front-line therapy between 1986 and 2016, and (B) according to the time of relapse (1986-2000 vs 2001-2016).
Rituximab became generally available for most patients with relapsed mature B-NHL after 2000, as were different reinduction regimens. To identify a trend in survival over time and define a suitable population for a risk factor analysis at relapse, the survival of relapsed patients was analyzed according to the time of relapse with a cutoff after 2000. Survival significantly improved from 11 ± 4% to 27 ± 5% after 2000 (P < .001; Figure 1B).
All further patient risk factor analyses were therefore restricted to the 75 patients with relapse of a BL/B-AL after 2000.
Patient characteristics
The patient characteristics of all 157 patients with progression of a BL/B-AL and the 75 diagnosed from 2001 are shown in Table 1. Distribution of age and gender reflects the typical distribution of BL/B-AL in children and adolescents. The progressions/relapses occurred very early, a median of 0.4 years after start of initial therapy (Table 1). More than one-third of the patients progressed during initial therapy. Bone marrow and CNS involvement were detected in one-third of the patients at relapse, respectively. Given the lower relapse rate in the lower risk groups, patients with initial higher stages and front-line treatment in risk groups R3/R4 are overrepresented at relapse. The patient characteristics for relapsed patients are shown separately for patients with initial BL and B-AL in the supplemental Table 2.
Characteristics of 157 patients with progression or relapse of a BL/B-AL during or after BFM-type chemotherapy diagnosed between 1986 and 2016
| . | All . | Until 2000 . | From 2001 . | P . |
|---|---|---|---|---|
| All patients | 157 | 82 | 75 | |
| Sex | ||||
| Male | 127 (81%) | 66 (80%) | 61 (81%) | 1.0 |
| Female | 30 (19%) | 16 (20%) | 14 (19%) | |
| Median age at relapse, y | 9.9 (2.9-19.6) | 9.9 (2.9-19.6) | 9.6 (3.2-18.7) | .91 |
| Median time from diagnosis to relapse, y | 0.4 (0-2) | 0.4 (0-2) | 0.4 (0.1-2) | .63 |
| Progression/relapse | ||||
| During first-line | 81 (52%) | 53 (65%) | 28 (37%) | .001 |
| After first-line | 76 (48%) | 29 (35%) | 47 (63%) | |
| Initial stage* | ||||
| I | 2 (1%) | 2 (3%) | .53 | |
| II | 6 (4%) | 2 (2%) | 4 (5%) | |
| III | 61 (39%) | 33 (40%) | 28 (37%) | |
| IV | 20 (13%) | 12 (15%) | 8 (11%) | |
| B-AL | 64 (41%) | 35 (43%) | 29 (41%) | |
| NA | 4 (3%) | — | 4 (5%) | |
| CNS (initial) | ||||
| No | 117 (75%) | 61 (74%) | 56 (75%) | 1.0 |
| Yes | 40 (25%) | 21 (26%) | 19 (25%) | |
| Initial therapy branch | ||||
| R1/R2 | 18 (12%) | 4 (5%) | 14 (19%) | .01 |
| R3/R4 | 137 (88%) | 76 (95%) | 61 (81%) | |
| BM-relapse | ||||
| No | 98 (62%) | 49 (60%) | 49 (65%) | .51 |
| Yes | 59 (38%) | 33 (40%) | 26 (35%) | |
| CNS relapse | ||||
| No | 115 (73%) | 62 (76%) | 53 (71%) | .59 |
| Yes | 42 (27%) | 20 (24%) | 22 (29%) | |
| Local relapse | ||||
| No | 94 (60%) | 54 (66%) | 40 (53%) | .14 |
| Yes | 63 (40%) | 28 (34%) | 35 (47%) | |
| Other relapse | ||||
| No | 115 (73%) | 66 (80%) | 49 (65%) | .05 |
| Yes | 42 (27%) | 16 (20%) | 26 (35%) |
| . | All . | Until 2000 . | From 2001 . | P . |
|---|---|---|---|---|
| All patients | 157 | 82 | 75 | |
| Sex | ||||
| Male | 127 (81%) | 66 (80%) | 61 (81%) | 1.0 |
| Female | 30 (19%) | 16 (20%) | 14 (19%) | |
| Median age at relapse, y | 9.9 (2.9-19.6) | 9.9 (2.9-19.6) | 9.6 (3.2-18.7) | .91 |
| Median time from diagnosis to relapse, y | 0.4 (0-2) | 0.4 (0-2) | 0.4 (0.1-2) | .63 |
| Progression/relapse | ||||
| During first-line | 81 (52%) | 53 (65%) | 28 (37%) | .001 |
| After first-line | 76 (48%) | 29 (35%) | 47 (63%) | |
| Initial stage* | ||||
| I | 2 (1%) | 2 (3%) | .53 | |
| II | 6 (4%) | 2 (2%) | 4 (5%) | |
| III | 61 (39%) | 33 (40%) | 28 (37%) | |
| IV | 20 (13%) | 12 (15%) | 8 (11%) | |
| B-AL | 64 (41%) | 35 (43%) | 29 (41%) | |
| NA | 4 (3%) | — | 4 (5%) | |
| CNS (initial) | ||||
| No | 117 (75%) | 61 (74%) | 56 (75%) | 1.0 |
| Yes | 40 (25%) | 21 (26%) | 19 (25%) | |
| Initial therapy branch | ||||
| R1/R2 | 18 (12%) | 4 (5%) | 14 (19%) | .01 |
| R3/R4 | 137 (88%) | 76 (95%) | 61 (81%) | |
| BM-relapse | ||||
| No | 98 (62%) | 49 (60%) | 49 (65%) | .51 |
| Yes | 59 (38%) | 33 (40%) | 26 (35%) | |
| CNS relapse | ||||
| No | 115 (73%) | 62 (76%) | 53 (71%) | .59 |
| Yes | 42 (27%) | 20 (24%) | 22 (29%) | |
| Local relapse | ||||
| No | 94 (60%) | 54 (66%) | 40 (53%) | .14 |
| Yes | 63 (40%) | 28 (34%) | 35 (47%) | |
| Other relapse | ||||
| No | 115 (73%) | 66 (80%) | 49 (65%) | .05 |
| Yes | 42 (27%) | 16 (20%) | 26 (35%) |
BM, bone marrow; NA, not available because of missing initial lumbar puncture.
According to St. Jude’s staging system.
Reinduction approaches, response to reinduction, and SCT
After exclusion of 1 patient without treatment information, 1 who died immediately at diagnosis of relapse, and 2 who received palliative care at relapse, information on reinduction and SCT approaches with curative intent were available for 71 relapse patients diagnosed after 2000.
The reinduction chemotherapy given varied considerably (Table 2). The reinduction regimens were grouped according to the first reinduction chemotherapy course because most second progressions were observed already after the first course. Twenty-seven patients were treated by the BFM-type front-line courses for high-risk patients as recommended in protocol NHL-BFM95 (CC, AA, or BB courses),5 of whom 16 received rituximab during reinduction. Ten patients were reinduced by R-ICE, 6 by R-ICI or ICN, and 4 by dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine, prednisone, and rituximab without radiation therapy (EPOCH-R).11,21 The clinical observations of both a high treatment-related mortality and high rate of further progression during reinduction in the 1990s led to refinements of the reinduction approach in the early 2000s. Since 2003, therefore, a strategy addressing the aggressive biology of BL/B-AL and the reduced treatment tolerance of children early after intensive front-line therapy was followed by a few centers. Two to 3 highly intensive continuous-infusion chemotherapy courses without high-dose methotrexate were applied with very short intervals, often not waiting for bone marrow regeneration. This time intensity did not allow for collection of autologous stem cells, therefore necessitating an allogeneic SCT for consolidation. The courses R-VICI (supplemental Figure 1), followed by a second R-VICI or R-ICI/NT, include drugs with activity in BL, bearing a relatively low risk of mucositis and having shown to act synergistically with rituximab. This approach was used in 15 patients at the diagnosis of the first relapse. Different other regimens were applied to 11 patients. Rituximab (55) or other CD20-antibodies (3) were given to all but 13 patients during reinduction.
Reinduction therapies, stem cell transplantations, treatment-related deaths, relapse, and survival for 75 patients* with relapsed BL/B-AL between 2001 and 2016
| Reinduction . | CC/AA-based . | R-ICE . | R-ICI/ICN . | VICI/ICIT . | EPOCH . | Other . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | 27 | 10 | 6 | 15 | 4 | 11 | ||||||
| Rituximab | 16 (/25) | 10 | 6 | 15 | 4 | 9 | ||||||
| TRD before SCT | 0 | 0 | 0 | 1 | 0 | 0 | ||||||
| Progression before SCT | 13 | 6 | 3 | 3 | 2 | 8 | ||||||
| SCT | 20* | 6* | 3 | 11 | 2 | 4 | ||||||
| Kind of SCT | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo |
| 12† | 8† | 4 | 2† | 1 | 2 | 0 | 11 | 0 | 2 | 3 | 1 | |
| TRD after SCT | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Relapse after SCT | 8 | 3 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 1 | ||
| Survival | 2 | 4* | 2‡ | 0 | 0 | 1 | 10 | 1 | 1 | 0 | ||
| Reinduction . | CC/AA-based . | R-ICE . | R-ICI/ICN . | VICI/ICIT . | EPOCH . | Other . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | 27 | 10 | 6 | 15 | 4 | 11 | ||||||
| Rituximab | 16 (/25) | 10 | 6 | 15 | 4 | 9 | ||||||
| TRD before SCT | 0 | 0 | 0 | 1 | 0 | 0 | ||||||
| Progression before SCT | 13 | 6 | 3 | 3 | 2 | 8 | ||||||
| SCT | 20* | 6* | 3 | 11 | 2 | 4 | ||||||
| Kind of SCT | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo |
| 12† | 8† | 4 | 2† | 1 | 2 | 0 | 11 | 0 | 2 | 3 | 1 | |
| TRD after SCT | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Relapse after SCT | 8 | 3 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 1 | ||
| Survival | 2 | 4* | 2‡ | 0 | 0 | 1 | 10 | 1 | 1 | 0 | ||
allo, allogeneic SCT; auto, autologous SCT; CR, complete remission; TRD, treatment-related death.
Exclusion: 1 patient without data on treatment, 1 patient who died immediately at relapse, 2 patients who received palliative care.
SCT after second relapse/progression during reinduction: CC: 3 patients (2× auto - death of disease, 1× allo - CR), ICE: 2 patients (2× allo - death of disease).
One patient: isolated testicular relapse.
About one-half of the patients experienced a second progression during reinduction with any regimen but R-VICI, during which 20% (3/15) progressed (Table 2). One patient died of Candida glabrata sepsis after the first R-VICI course; no further therapy-associated deaths occurred during reinduction before SCT (Table 2).
SCT was intended for all 71 patients. Altogether, 46 of the 71 patients (65%) received the intended SCT, 41 after the first relapse and 5 after a second progression. Twenty patients were consolidated by an autologous SCT (18 after first relapse, 2 after a second progression) and 26 by an allogeneic SCT (23 after first relapse, 3 after a second progression). There was a correlation of reinduction regimen and type of SCT applied (Table 2). Whereas about two-thirds of the patients reached SCT after reinduction by BFM-type frontline courses (12/20), R-ICE (4/6), or other regimen (3/4) were consolidated by an autologous SCT, more patients received an allogeneic SCT after R-ICI/ICN (2/3), as did the 2 patients after dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine, prednisone, and rituximab without radiation therapy and all 11 patients transplanted after reinduction with R-VICI courses. This correlation precludes a direct comparison of the efficacy of the type of transplantation and allows for comparison of the different strategies only.
High-dose therapy used before autologous SCT was the recommended busulfan-containing regimen (supplemental Figure 1) in 16 patients. One patient received carmustine, etoposide, cytarabine, and melphalan; the conditioning regimen was not known for 3 patients (Table 2).
Several conditioning regimens were used for allogeneic SCT. Busulfan-based myeloablative regimen were applied in 6 patients, 1 patient received a treosulfan-based regimen, and 3 patients 12 Gy total body irradiation. Because the busulfan-based conditioning regimen suggested in the NHL-BFM95 study was not effective and toxic for the majority of patients, a reduced-intensity conditioning (RIC) regimen was adapted to the needs of patients with BL relapse, including fludarabine and thiotepa to ensure engraftment augmented by drugs effective against BL. This RIC regimen was given to 17 patients (Table 2; supplemental Figure 1). Again, there was a correlation of the overall treatment strategy for relapse with the type of conditioning regimen used for allogeneic SCT. Fourteen patients who received R-VICI-type reinduction courses as treatment of first relapse (10) or second progression (4) were conditioned by the new RIC regimen.
Stem cell donors were known for 23 patients. Five patients received grafts from matched sibling donors, 16 from matched unrelated donors (9/10 or 10/10 HLA match), 1 from a haploidentical, and 1 from a mismatched unrelated donor.
Patient characteristics and treatment modalities associated with survival
We analyzed the association of initial and relapse characteristics, time of relapse, and therapy at relapse (reinduction, rituximab, and type of SCT) with the outcome of the patients with relapsed BL/B-AL diagnosed after 2000.
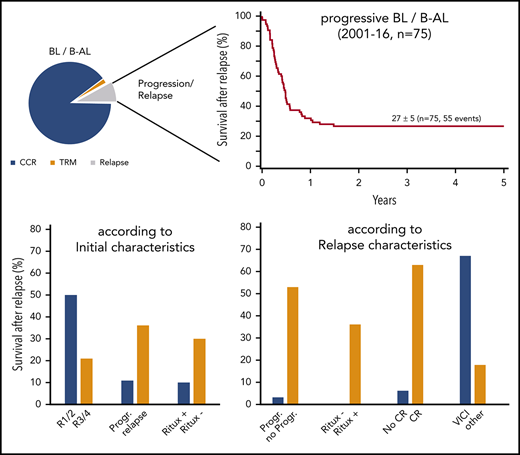
Regarding association of first-line therapy parameters and survival in relapse, outcome was poor for patients with progression during initial therapy (25 of 28 died) or patients who had front-line rituximab (9 of 10 died) (Table 3). Although not statistically different, only 2 of 20 patients with initial CNS involvement survived after relapse (10 ± 7%) compared with 33 ± 6% without initial CNS disease (P = .13). Survival of 14 patients with relapse after initial therapy for low-risk disease (R1/R2) was 50 ± 13% compared with 21 ± 5% for 61 patients relapsing after R3/R4 therapy (P < .02) (Figure 2A).
Risk factor analysis for survival at relapse among 75 patients with relapsed BL/B-AL diagnosed between 2001 and 2016: univariate analysis
| Characteristics . | Patients, n . | Event, n . | Survival at 4 y, % . | P . |
|---|---|---|---|---|
| Initial CNS involvement | ||||
| Positive | 20 | 18 | 10 ± 7 | .13 |
| Negative | 55 | 37 | 33 ± 6 | |
| Progression/relapse during initial therapy | ||||
| Yes | 28 | 25 | 11 ± 7 | .001 |
| No | 47 | 30 | 36 ± 7 | |
| Initial therapy branch | ||||
| R1/R2 | 14 | 7 | 50 ± 13 | .017 |
| R3/R4 | 61 | 48 | 21 ± 5 | |
| Rituximab during initial therapy (n = 70) | ||||
| No | 60 | 42 | 30 ± 6 | .04 |
| Yes | 10 | 9 | 10 ± 9 | |
| CNS involvement at relapse | ||||
| Positive | 22 | 18 | 18 ± 8 | .56 |
| Negative | 53 | 37 | 30 ± 6 | |
| BM at relapse | ||||
| Positive | 26 | 18 | 31 ± 9 | .91 |
| Negative | 48 | 36 | 25 ± 6 | |
| Rituximab in relapse (n = 63) | ||||
| No | 13 | 13 | .016 | |
| Yes | 55 | 35 | 36 ± 6 | |
| Progression during re-induction (n = 68) | ||||
| No | 36 | 17 | 53 ± 8 | <.001 |
| Yes | 32 | 31 | 3 ± 3 | |
| CR before SCT (SCT n = 46) | ||||
| Yes | 30 | 11 | 63 ± 9 | <.001 |
| No | 16 | 15 | 6 ± 6 | |
| Reinduction regimen (n = 71) | ||||
| VICI | 15 | 5 | 67 ± 12 | .003 |
| All other | 56 | 46 | 18 ± 5 |
| Characteristics . | Patients, n . | Event, n . | Survival at 4 y, % . | P . |
|---|---|---|---|---|
| Initial CNS involvement | ||||
| Positive | 20 | 18 | 10 ± 7 | .13 |
| Negative | 55 | 37 | 33 ± 6 | |
| Progression/relapse during initial therapy | ||||
| Yes | 28 | 25 | 11 ± 7 | .001 |
| No | 47 | 30 | 36 ± 7 | |
| Initial therapy branch | ||||
| R1/R2 | 14 | 7 | 50 ± 13 | .017 |
| R3/R4 | 61 | 48 | 21 ± 5 | |
| Rituximab during initial therapy (n = 70) | ||||
| No | 60 | 42 | 30 ± 6 | .04 |
| Yes | 10 | 9 | 10 ± 9 | |
| CNS involvement at relapse | ||||
| Positive | 22 | 18 | 18 ± 8 | .56 |
| Negative | 53 | 37 | 30 ± 6 | |
| BM at relapse | ||||
| Positive | 26 | 18 | 31 ± 9 | .91 |
| Negative | 48 | 36 | 25 ± 6 | |
| Rituximab in relapse (n = 63) | ||||
| No | 13 | 13 | .016 | |
| Yes | 55 | 35 | 36 ± 6 | |
| Progression during re-induction (n = 68) | ||||
| No | 36 | 17 | 53 ± 8 | <.001 |
| Yes | 32 | 31 | 3 ± 3 | |
| CR before SCT (SCT n = 46) | ||||
| Yes | 30 | 11 | 63 ± 9 | <.001 |
| No | 16 | 15 | 6 ± 6 | |
| Reinduction regimen (n = 71) | ||||
| VICI | 15 | 5 | 67 ± 12 | .003 |
| All other | 56 | 46 | 18 ± 5 |
Survival at 4 years of 75 patients with relapse or progression of a BL or B-AL during or after appropriate risk-adapted NHL-BFM front-line therapy between 2001 and 2016. According to the initial therapy branch (A), and according to the reinduction regimen (B).
Survival at 4 years of 75 patients with relapse or progression of a BL or B-AL during or after appropriate risk-adapted NHL-BFM front-line therapy between 2001 and 2016. According to the initial therapy branch (A), and according to the reinduction regimen (B).
Among the clinical characteristics at relapse and relapse therapy parameters analyzed for their correlation with survival, 4 showed a significant association. All 13 patients who did not receive rituximab during reinduction died, as did 31 of 32 with progression during reinduction and 15 of 16 not reaching a CR before SCT (Table 3). The only patient who survived a second progression during reinduction experienced the first progression during initial therapy, relapsed again after 1 course of R-CC, was treated with 1 course of R-ICNT, which was followed directly by a matched sibling donor SCT after conditioning with the new RIC regimen.
The survival rate in relapse did not differ significantly between the BFM-type reinduction regimen with CC/AA (22 ± 8%), ICE (20 ± 12%), ICI/ICN (17 ± 15%), and others (8 ± 7%) followed by any type of SCT. Survival after the R-VICI type regimen followed by allogeneic transplantation was 67 ± 12% compared with 19 ± 5% for all other regimen and transplantation approaches (P = .004) (Table 3; Figure 2B).
Twenty-five of the 71 patients for whom SCT was intended did not receive a SCT because of progression during reinduction. They all died of lymphoma progression. Forty-six patients received SCT (20 autologous, 26 allogeneic). Survival was 25 ± 10% after autologous SCT compared with 58 ± 10% after allogeneic SCT (P = .026). The difference stayed comparable when focusing on the patients who were transplanted after a first relapse/progression only (28 ± 11% after autologous SCT; 65 ± 10% after allogeneic SCT, P = .007). However, the association of the reinduction regimen with the type of SCT precludes interpreting the comparison of the efficacy of the type of transplantation without taking into account the overall relapse strategy. Regarding the conditioning regimen for allogeneic SCT, all 4 patients receiving a busulfan- or treosulfan-based conditioning regimen for allogeneic SCT died, as did 2 of 3 patients after total body irradiation-based conditioning. Even though 3 of the 17 patients transplanted after the RIC regimen were transplanted after a second progression, 14 of the 17 patients survived, 2 relapsed, and 1 died from transplant complications. There was no association of the stem cell donor with survival among the patients who were consolidated by an allogeneic SCT (survival: matched sibling donor 3/5, matched unrelated donor 9/16, haplo-/mismatched unrelated donor 1 /2).
In multivariate analysis, we considered parameters showing a significant association with survival in univariate analysis but excluded response to reinduction and CR at SCT with the goal to focus on parameters that allow for formation of a relapse strategy. When considering time to relapse (after therapy vs during first-line therapy), initial risk group (R3/4 vs R1/2), front-line rituximab, rituximab during reinduction, and reinduction chemotherapy (VICI vs other), the initial risk group and reinduction chemotherapy retained an independent prognostic value (Table 4).
Risk factor analysis for survival at relapse among 75 patients with relapsed BL/B-AL diagnosed between 2001 and 2016: Cox regression analysis
| Characteristics . | HR (95% CI) . | P . |
|---|---|---|
| Time of relapse (after initial therapy) | 0.55 (0.29-1.02) | .058 |
| Initial therapy branch (R3/4) | 2.69 (1.1-6.5) | .027 |
| Rituximab front-line | 1.86 (0.82-4.22) | .14 |
| No rituximab in relapse | 0.79 (0.40-1.58) | .52 |
| Reinduction regimen (VICI) | 0.24 (0.09-0.64) | .004 |
| Characteristics . | HR (95% CI) . | P . |
|---|---|---|
| Time of relapse (after initial therapy) | 0.55 (0.29-1.02) | .058 |
| Initial therapy branch (R3/4) | 2.69 (1.1-6.5) | .027 |
| Rituximab front-line | 1.86 (0.82-4.22) | .14 |
| No rituximab in relapse | 0.79 (0.40-1.58) | .52 |
| Reinduction regimen (VICI) | 0.24 (0.09-0.64) | .004 |
Discussion
Current effective risk-adapted short-pulse chemotherapy regimens for BL and B-AL leave highly refractory relapsed disease. We analyzed for the first time a population-based large cohort of patients with BL/B-AL with progression during or after initial risk-adapted therapy of adequate intensity. This unique focus on the biologically defined entity allowed for an analysis of risk factors for children with BL/B-AL in relapse as well as analysis of the efficacy of different relapse therapies applied.
The survival of patients with relapsed or refractory BL stays 20% to 30% even after the year 2000 in our and other smaller cohorts with identifiable BL.6,7,10,12 The relapse characteristics of the patients with BL/B-AL in our series are comparable to earlier reports including all patients with mature B-NHL, with one-third of the patients showing progression during initial therapy and one-third each having bone marrow and CNS involvement at relapse.6,7,10,12
Among the initial clinical and therapy characteristics, we identified initial high-risk disease and progression during therapy as risk factors for survival in relapse. The latter is in line with all series on patients with relapsed mature B-NHL in which time to failure was shown to be an independent risk factor for survival when using a cutoff of 6 months from diagnosis.6,7,12 Because the risk stratification of initial therapy in the BFM studies includes LDH and stage, the findings of the impact of initial risk group on outcome of BL in relapse are in line with analyses of the French-American-British (FAB) LMB96 trial and a French series on all relapsed mature B-NHL.6,12
The addition of rituximab to front-line chemotherapy has been shown to increase the event-free survival of children with high-risk mature B-NHL and now is regarded as standard therapy for children with high-risk BL/B-AL.17,22,23 Our observation that only 1 of the patients with relapsed BL/B-AL who received front-line rituximab could be salvaged might indicate that the few remaining relapses among children with high-risk BL/B-AL in the future are even more resistant.
In contrast to the analysis of all progressive or relapsed B-NHL from the FAB LMB96 trial, bone marrow involvement at relapse was not a risk factor among BL/B-AL patients in our analysis.6 This might be explained by the observation that patients with diffuse large B-cell lymphoma or primary mediastinal large B-cell lymphoma who have a significantly better outcome in relapse compared with patients with BL in most studies rarely have involvement of BM even at relapse.1,3,5,12,24
Response to reinduction, receiving an SCT, and remission status at SCT were associated with survival in our cohort of relapsed BL patients, with almost no chance to survive for patients with progression during reinduction or those not reaching a remission before SCT. This is in line with earlier retrospective analyses on all mature B-NHL from several study groups as well as the prospective study on R-ICE reinduction.7,10,11,13 Given that SCT was intended for almost all patients in our cohort, these data underscore that the efficacy of the first reinduction approach is essential to providing a chance of cure. Based on these observations, it is of utmost importance for patients with early relapsing or refractory BL/B-AL to receive effective reinduction regimens for rapid induction of CR that remain tolerable for these heavily pretreated children.
Even though the number of patients treated with the different reinduction regimen after the year 2000 is limited in our analysis and keeping in mind its retrospective nature, our data suggest that frontline rituximab B-NHL courses, R-ICE, R-ICI, or EPOCH-R, have limited efficacy in relapsed BL with progressions during reinduction in one-half of patients and survival rates ≤20%. This is in line with the observation of other retrospective analyses and the only prospective trial testing R-ICE.7,11-13 The addition of rituximab to the reinduction of rituximab-naïve BL-relapse patients was essential to open a possibility for cure in our and a British analysis including all relapsed mature B-NHL.7 Given the medical need to address the aggressive biology of relapsed BL/B-AL and to take advantage of a possible synergistic effect of rituximab with chemotherapeutic drugs, a continuous-infusion, time-, and dose-condensed reinduction regimen was used, also including drugs not usually used against BL front-line.25,26 Furthermore, keeping in mind the reduced treatment tolerance of the children early after intensive front-line therapy, especially regarding mucositis, a methotrexate-free regimen was chosen. We applied R-VICI followed by a second course R-VICI or R-ICI/NT initially at 1 center for a few patients. Because the intended time intensity did not allow for the collection of autologous stem cells, the overall approach necessitated an allogeneic SCT for consolidation, even though the available data on SCT for mature B-NHL do not imply an advantage of allogeneic over autologous SCT.7,12,15 After the first patients with relapses of high-risk BL/B-AL survived, this approach has been followed by several centers in 15 patients relapsing during or after BFM frontline therapy since 2003. The high survival of the patients of >50% after this approach greatly exceeds the survival observed after all other regimens and SCT approaches. This led to the inclusion of this backbone into the only recruiting clinical study for children with relapsed BL/B-AL testing ibrutinib in addition to reinduction (NCT02703272). This overall approach should form the basis for a backbone for international studies testing new drugs in relapsed BL/B-AL.27 Antibody–drug conjugates and bispecific antibodies emerge as promising available drugs to be tested together with reinduction. Because the production of chimeric antigen receptor T cells currently takes more than 4 weeks, these drugs may be available for consolidation rather than reinduction. The latter may change with the generation of “off-the-shelf chimeric antigen receptor T cells.”27
The reinduction regimen R-VICI was applied additionally to 6 patients who progressed during reinduction with other regimens (data not shown). With 1 exception, R-VICI did not prevent further progressions and death. This observation again underlines that the aggressiveness of relapsed BL necessitates choosing a very intensive and effective first reinduction course and does not allow starting with a more subtle approach with the intention to test individual treatment tolerability. The question remains open whether the efficacy of R-VICI and the described overall approach may be applicable to children who receive rituximab front-line. Of the 2 rituximab-pretreated children who were treated by R-VICI in relapse, 1 died of progressive disease and the other survived.
In light of the poor survival of children with relapsed BL/B-AL, all efforts should be undertaken to characterize the relapsed tumors molecularly to detect possible targetable lesions, despite the overall low number of genetic alterations in BL/B-AL, and enable detection of these patients already in initial therapy.28
In summary, our data on a large cohort of children with relapsed BL/B-AL after current effective front-line therapy show that initial high-risk disease and progression during initial therapy as well as pretreatment with rituximab are associated with very poor survival in relapse. Progression during reinduction or not reaching a remission before consolidation by SCT was almost uniformly fatal, therefore asking for experimental and conceptional different approaches not only for children with second relapse but also for patients not reaching remission during a first reinduction approach. Time-condensed continuous-infusion reinduction with an anti-CD20 antibody followed by SCT should form the basis for testing new drugs. Given the very low number of relapsed patients in each country and availability of new drugs, an international collaborative platform offering the possibility to rapidly test different available new approaches for children with relapsed BL/B-AL is currently established.27
Presented in part at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 6 December 2014.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
For access to deidentified participant data, please contact the corresponding author via e-mail.
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the expert work of Ulrike Meyer and Nora Muehlegger (data management) and thank the other members of the national reference pathology panel in addition to I.O. and W.K. (A.C. Feller, M.L. Hansmann, P. Möller, H. Müller-Hermelink, H. Stein). The authors especially thank the doctors, nurses, and data managers in participating hospitals of the Non-Hodgkin’s Lymphoma-Berlin-Frankfurt-Münster (NHL-BFM) study group for their care for the patients and for supply of data.
The NHL-BFM Registry is supported by grants DKS 2014.11A/B and DKS 2016.24A/B from the Deutsche Kinderkrebsstiftung (B.B. and W.W.) and Forschungshilfe Peiper (A.M. and A.R.).
Authorship
Contribution: W.W., M.Z., A.R., and B.B. designed the study; W.W., A.M., S.M., H.H., F.K., F.N., E.K., A.A., A.R., and B.B. contributed patients and data; I.O. and W.K. performed reference histopathology; W.W., M.Z., A.R., and B.B. analyzed the data; W.W. and M.Z. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wilhelm Woessmann, NHL-BFM Study Center and Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Building O45, 20246 Hamburg, Germany; e-mail: w.woessmann@uke.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal