Key Points
Using a systematic approach, we identified 23 prognostic factors for venous thromboembolism and 15 for bleeding.
We identified several prognostic factors for VTE and bleeding that are not considered in most of the widely used risk assessment models.
Abstract
There may be many predictors of venous thromboembolism (VTE) and bleeding in hospitalized medical patients, but until now, systematic reviews and assessments of the certainty of the evidence have not been published. We conducted a systematic review to identify prognostic factors for VTE and bleeding in hospitalized medical patients and searched Medline and EMBASE from inception through May 2018. We considered studies that identified potential prognostic factors for VTE and bleeding in hospitalized adult medical patients. Reviewers extracted data in duplicate and independently and assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation approach. Of 69 410 citations, we included 17 studies in our analysis: 14 that reported on VTE, and 3 that reported on bleeding. For VTE, moderate-certainty evidence showed a probable association with older age; elevated C-reactive protein (CRP), D-dimer, and fibrinogen levels; tachycardia; thrombocytosis; leukocytosis; fever; leg edema; lower Barthel Index (BI) score; immobility; paresis; previous history of VTE; thrombophilia; malignancy; critical illness; and infections. For bleeding, moderate-certainty evidence showed a probable association with older age, sex, anemia, obesity, low hemoglobin, gastroduodenal ulcers, rehospitalization, critical illness, thrombocytopenia, blood dyscrasias, hepatic disease, renal failure, antithrombotic medication, and presence of a central venous catheter. Elevated CRP, a lower BI, a history of malignancy, and elevated heart rate are not included in most VTE risk assessment models. This study informs risk prediction in the management of hospitalized medical patients for VTE and bleeding; it also informs guidelines for VTE prevention and future research.
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), has an annual incidence of ∼1 per 1000 in adult populations.1,2 VTE is a major burden in hospitalized medical patients. Medical patients can be classified as having acute, critical, or chronic medical illness, and their risk for both VTE and bleeding may depend on the severity of their medical illness. The incidence of VTE in hospitalized acutely ill medical patients detected by screening is up to 14.9%.3 From 50% to 70% of symptomatic VTEs and 70% to 80% of fatal PEs occur in acute medically ill patients.4-7
The risk of hospital-acquired VTE is reduced by using pharmacological and nonpharmacological interventions, but these interventions are not without potential patient harms. Risk assessment models (RAMs) have been used in hospitalized medically ill patients to stratify the different subsets of patients by their risk of having a VTE or clinically significant bleeding event.8 This stratification may then support optimized management for the prevention of either outcome.9 A RAM is a formal combination of multiple predictors from which risks of a specific end point can be calculated for individuals. The value of using RAMs include generation of specific baseline risks to inform recommendations for a strata of patients and calculation of a predicted risk of an outcome for an individual patient (eg, VTE or bleeding) based on the patient’s characteristics (ie, the prognostic factors). Implementation of RAMs in the field of VTE prevention can be accomplished by embedding them in clinical encounters or decision aids to individualize the use of guideline recommendations. However, the application of RAMs is variable in current practice.3
Most RAMs are developed using data registries that are not based on a systematic review of all potential prognostic factors.10 However, guiding principles for developing RAMs describe the importance of identifying prognostic factors through systematic reviews.10 We identified only one systematic review, conducted 11 years ago, that evaluated VTE as an outcome in medical patients, but the effect sizes of the prognostic factors were not subjected to meta-analysis, and bleeding risk, critical for balancing benefits and harms in these patients, was not included as an outcome.11
Therefore, our aim was to conduct a systematic review of prognostic factors for VTE and bleeding in hospitalized medical patients that may inform management, future guideline recommendations, and the development of RAMs in hospitalized medical patients.
Methods
We conducted a systematic review using Cochrane methodology to identify studies that reported on prognostic factors for VTE and bleeding in hospitalized medical patients.12 We developed a protocol that was reviewed and revised by the coauthors, but we did not register it because of confidentiality clauses in the research contract.
Data sources and searches
We searched Medline and EMBASE from inception through May 2018 with the assistance of an information scientist. Supplemental Table 1 (available on the Blood Web site) provides detailed descriptions of the search strategy. The search included both medical subject heading (MesH) terms and text-word terms. It combined VTE-related terms with primary prevention terms and 2 search blocks defining prognosis and prediction guide filters. We used no language restrictions or time limits.
Study selection
Four teams of 2 reviewers participated in training and calibration exercises before starting the screening processes. The teams screened independently and in duplicate the titles and abstracts of all the retrieved citations. They then retrieved the full texts of all citations judged as potentially eligible by at least 1 of the reviewers on each team. The reviewers screened the full texts independently and in duplicate and compared their results. A third senior reviewer resolved disagreements when necessary. Reviewers used a standardized screening form and conducted calibration exercises before the screening process. The eligibility criteria for study selection comprised the following characteristics.
Population
We included studies that evaluated adult medical patients who were acutely, critically, or chronically ill. We also included studies in which the population included nonmedical patients or medical patients with a recent history of surgery or trauma if the final regression model adjusted for these factors. We included studies if less than 10% of the population was receiving thromboprophylaxis or if the statistical analysis adjusted for the use of thromboprophylaxis. Thromboprophylaxis included the use of anticoagulation therapy (ie, warfarin, low-molecular-weight heparin, and unfractionated heparin), antiplatelet therapy (ie, aspirin), or mechanical prophylaxis (ie, elastic stockings or intermittent pneumatic compression).
We excluded studies if the population did not reflect the general population of interest, such as studies of only selected types of cancer patients.13,14 We defined acutely ill medical patients as those hospitalized for a medical illness including heart failure, respiratory insufficiency, stroke, and infectious or inflammatory diseases requiring urgent care.2 Critically ill patients were those who had an immediately life-threatening condition and were admitted to an intensive or critical care unit.2 Chronically ill medical patients included those with acute exacerbations of chronic medical conditions who required hospitalization.2
Exposure
We investigated all prognostic factors reported in individual studies.
Comparisons
We investigated the absence or different levels of the prognostic factor.
Outcomes
Studies had to report on the outcomes VTE or bleeding. VTE was defined as any symptomatic or asymptomatic DVT or PE within 90 days after discharge. Bleeding included major or nonmajor but clinically significant bleeding within 90 days after discharge.8
Setting
Studies that included patients who were admitted to a nonsurgical inpatient ward.
Type of study
Data extraction
Two reviewers abstracted data independently and in duplicate from all eligible studies using standardized forms. Reviewers compared and discussed results and consulted a third reviewer in case of any disagreement. We conducted calibration exercises and piloting of all forms before the start of the data abstraction process. All eligible studies were published in English.
For all identified studies, RAMs, and prognostic factor studies, the reviewers abstracted data on the following characteristics:
Study context (eg, country and year of publication)
Type of prediction model study (development, validation, and impact)
Study design (eg, cohort or case-control; duration of follow-up)
Population and their demographics (eg, sample size, age, number of centers, and administration of prophylaxis and what type)
Outcomes (VTE and bleeding)
Prognostic factors, definitions, and measurement methods (including thresholds used for continuous predictors)
Measures of association (eg, odds ratio [OR], risk ratio, and hazard ratio)
Quality assessment
Risk-of-bias assessment
Synthesis of findings and certainty of evidence assessment
We presented the results of the studies, including the individual prognostic factors, in both tabular and narrative formats. We also described the identified prognostic factor studies and the measure of association with the outcomes of interest. We performed an assessment of the certainty of evidence for each of the prognostic factors per outcome, based on the GRADE approach.15 The approach considers the following domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. We developed evidence profiles and rated the overall certainty of evidence as high, moderate, and low or very low, depending on the grading of the individual domains.15 We narratively described the strength of the association using the terms “there is,” “there probably is,” or “there may be,” depending on whether the quality of the evidence was “high,” “moderate,” or “low/very low,” respectively.
Data synthesis and analysis
We standardized the units of measurement for each prognostic factor, unifying the direction of the predictors, adjusting the weights of the studies, and calculating crude-effect estimates when not provided.21 When possible, we performed meta-analysis of all prognostic factors associated with the outcomes VTE and bleeding that were reported by more than 1 study. We then presented the effect estimate as OR and the corresponding 95% confidence interval (CI). In studies that reported the measure of association as an hazard ratio or risk ratio, we converted them to ORs using the baseline risk (incidence of those not on prophylaxis having VTE or bleeding out of the total sample) reported in the studies.22,23 We conducted a meta-analysis of associations using the generic inverse variance-based method to produce an overall measure of association. We used the crude effect estimates when the adjusted estimates were not provided. We explored consistency of the associations between the results of our meta-analysis and studies reporting the same predictors that could not be pooled. All analyses used random-effect models applying the prognosis module in Review Manager, version 5.3.24
Results
Figure 1 is a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. Our search identified 69 410 citations, of which we included 807 studies for full text assessment. Seventeen studies fulfilled the inclusion criteria for evaluating VTE or bleeding outcomes or both.7,8,25-39
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart.
Description of included studies
Table 1 describes the characteristics of the included studies reporting on the outcomes VTE and/or bleeding. Eight studies were prognostic factor studies: 4 were prognostic model development studies, and 5 were external validation studies. Five studies were retrospective case-control studies,25-29 2 of which were multicenter25,26 ; 5 were retrospective cohorts,30-34 3 of which were multicenter.31,32,34 Seven studies were prospective cohorts,7,8,35-39 4 of which were multicenter.7,8,36,39 The included studies were conducted in the United States (n = 9), China (n = 3), Canada (n = 2), United Kingdom (n = 1), Poland (n = 1), and Japan (n = 1). Of the 14 included studies for VTE (defined as proximal DVT or PE), 9 reported on symptomatic VTE only.7,25-31,34 The other 5 studies reported on both symptomatic and asymptomatic VTE.35-39 The follow-up time was up to 3 months in 12 of 14 studies reporting on VTE. The other 2 studies, Zhou et al29 and Yi et al,39 had a follow-up time of 6 months and 1 year, respectively, but also reported the occurrence of VTE during hospitalization. In accordance with our protocol, we used the incidence of VTE during hospitalization from those studies. The 3 studies that reported on bleeding (major or clinically relevant) had a follow-up time up to 1 month.8,32,33 Of the 14 studies reporting on VTE, 12 studies included patients who received thromboprophylaxis in 0.4% to 67% of the patients. Of those, 2 studies35,36 included less than 10% of patients on thromboprophylaxis, and 10 studies adjusted for prophylaxis in the statistical analysis.7,26-31,34,38,39 As for bleeding, all 3 studies reported prophylaxis use in 9% to 70%, which was accounted for in their analysis.8,32,33
Study characteristics
| First author, year (country) . | Population (sample size) . | Time frame (y) . | Mean age, y (SD)* . | Study type (number of centers and study design) . | Prophylaxis (%) . | Outcome (number of events) . | Diagnostic methods . | Follow-up time . | Variables in multivariate logistic regression, when applicable . |
|---|---|---|---|---|---|---|---|---|---|
| Venous thromboembolism | |||||||||
| Spyropoulos et al7 (US) | Acutely ill medical patients (N = 15 156) | 2002-2006 | 68 (52-79)* | Prognostic model development (multicenter prospective cohort) | VTE prophylaxis (adjusted-dose warfarin, elastic stockings, LMWH, unfractionated heparin, intermittent pneumatic compression, and aspirin): 44% | Any symptomatic VTE (lower extremity DVT, and PE) (n = 184) | Clinically observed VTE | 92 d | IMPROVE RAM-related factors: |
| The model was adjusted for VTE prophylaxis. | DVT verified by positive venogram or compression ultrasonography. | Age >60 y; prior cancer, prior VTE, ICU/CCU stay, lower limb paralysis, immobility | |||||||
| PE verified by positive lung scan, pulmonary angiogram, or spiral CT scan. | Known thrombophilia | ||||||||
| Fatal PE was defined as PE diagnosed at autopsy or, in the absence of autopsy, when PE was considered the most likely cause of death. | |||||||||
| Mahan et al25 (Canada) | Acute medical patients (N = 417. VTE cases: n = 139; non-VTE controls: n = 278) | 2005-2011 | Cases: 68; controls: 65 | External validation (multicenter retrospective case-control) | VTE prophylaxis, 0% | Any symptomatic VTE (lower extremity DVT, and PE; n = 139) | PE, verified by a positive pulmonary angiogram, spiral computed tomography, or high-probability ventilation/perfusion scan or at autopsy. | 92 d | IMPROVE RAM-related factors: |
| Lower extremity DVT verified by positive compression ultrasonography, computed tomography, or magnetic resonance imaging, or at autopsy. | Previous VTE; known thrombophilia; lower limb paralysis; current cancer; immobilization ≥7; ICU/CCU stay; age >60 y | ||||||||
| Rosenberg et al26 (US) | Medical patients (N = 539: VTE cases: n = 135 and non-VTE controls: n = 404) | 2009-2013 | 67 | External validation (multicenter retrospective case-control) | Any prophylaxis in VTE cases: 49% (of those 44% pharmacological VTE and 5% mechanical) | Any symptomatic VTE (n = 135) | VTE events identified using ICD-9 codes | Within 90 d following the index admission | IMPROVE RAM-related factors: same as those in Spyropoulos et al7 |
| Any prophylaxis in non-VTE controls: 45% (of those 40% pharmacological VTE and 5% mechanical) | |||||||||
| The results were essentially unchanged when the cases and controls were stratified into groups that received VTE prophylaxis, including pharmacological prophylaxis during hospitalization and those that did not. | |||||||||
| Zakai et al27 (US) | Patients admitted to medical services (N = 900: VTE cases: 299; and non-VTE controls: 601) | 2002-2009 | cases: 6317 ; controls: 6615 | Prognostic model development (single-center retrospective case-control) | Pharmacological prophylaxis in VTE cases: 64.6% and in non-VTE controls: 62.2%; | Any symptomatic VTE (upper and lower extremity DVT and PE; n = 299) | VTE events identified using ICD-9 VTE discharge codes. | Discharge or transfer from medical service | Venous thrombosis prophylaxis (mechanical; pharmacologic); demographics (age, sex, BMI); medical history (myocardial infarction, COPD, diabetes, chronic kidney disease); conditions active on admission (fever, COPD, pneumonia, any infection). |
| mechanical prophylaxis in VTE cases: 31.8% and in non-VTE controls: 27.6%; | Codes confirmed by clinician review. | ||||||||
| the model was adjusted for both mechanical and pharmacological prophylaxis. | Records were reviewed by a research nurse and all hospital-acquired VTE cases and 20% of noncases were reviewed by a physician. | ||||||||
| Zakai et al28 (US) | Medical patients (N = 188: VTE cases: 65 and non-VTE controls: 123) | 2000-2002 | 68 | External validation (single-center retrospective case-control) | VTE prophylaxis (including warfarin, unfractionated heparin, low molecular weight heparin or intermittent pneumatic compression devices): in VTE cases: 59%; and non-VTE controls 47%. | Any symptomatic VTE (upper and lower extremity DVT and PE; n = 65) | VTE events identified using ICD-9 VTE discharge codes. | LOS case: 16 (10-28); controls: 6 (4-10) | Trauma last 3 mo; active cancer past year; admission fever; leg edema on admission; immobility >72 h; bacterial infection (cellulitis, pneumonia, sepsis, other); platelet count >350 × 109/L; use of VTE prophylaxis |
| The model was adjusted for prophylaxis. | |||||||||
| Zhou et al29 (China) | Medically ill patients (N = 1804: VTE cases: 902 and non-VTE controls: 902) | 2013-2016 | Cases: 6017 ; controls: 5717 | External validation (single-center retrospective Case-control) | Any prophylaxis: in VTE Cases: 4.1% and in non-VTE controls: 6.1% | Any symptomatic VTE (defined as DVT or PE; n = 902) | DVT verified by positive compression ultrasonography and/or contrast venography. | 6 mo after discharge | Caprini RAM factors* |
| VTE prophylaxis included any mechanical use (intermittent pneumatic compression devices or sole vein pump) or pharmacological use (unfractionated heparin, LMWH, warfarin, fondaparinux sodium, etc.) | PE verified by positive pulmonary angiogram, spiral computed tomography, or high probability ventilation/perfusion scanning or at autopsy. | Padua RAM factors† | |||||||
| The model was adjusted for VTE prophylaxis | |||||||||
| Barclay et al30 (US) | Chronic Liver disease (N = 1581) | 2008-2011 | 51(11) | Prognostic factor (single-center retrospective cohort) | Pharmacological VTE prophylaxis: 24.8% (Unfractionated heparin: 9.7%; low molecular weight heparin: 88.0%; or both: 2.3%) | Any symptomatic VTE (including DVT, PE or portal vein thrombosis-PVT) (n = 23) | VTE event identified in the medical record. | 4-7 d | VTE prophylaxis |
| The model was adjusted for pharmacological prophylaxis | VTE confirmed with radiologic testing. | Active malignancy | |||||||
| Trauma or surgery during hospitalization | |||||||||
| History of VTE | |||||||||
| Grant et al31 (US) | Hospitalized medical patients (N = 63 548) | 2011-2014 | 66 | External validation (multicenter retrospective cohort) | Pharmacological VTE prophylaxis: 60.9% | Any symptomatic VTE (defined as proximal upper- or proximal lower-extremity DVT and PE) (n = 670) | VTE was clinically suspected. | 90 d | Caprini RAM factors* |
| The model was adjusted for the pharmacological prophylaxis | VTE was Image confirmed. | ||||||||
| Majority of events were identified by medical record review, 44 (6.6%) of the events were confirmed via telephone follow-up. | |||||||||
| Rothberg et al34 (US) | Medical Patients (N = 46 503) | 2004-2005 | NR | Prognostic model development (multicenter retrospective cohort) | Pharmacological VTE prophylaxis: 30% | Any symptomatic VTE (n = 1 052) | VTE verified by lower extremity ultrasonography, venography, CT angiogram, ventilation/perfusion scan, or pulmonary angiogram) on hospital day 3 or later | 30 30 d | Any prophylaxis; female; length of stay ≥6 d; age (18-49; 50-64, and >65 y); primary diagnosis (pneumonia, COPD, stroke, congestive heart failure, urinary tract infection, respiratory failure, septicemia) |
| There was no difference in the model estimates for the factors when the model was adjusted for prophylaxis. | Secondary diagnosis of VTE provided using ICD-9 diagnoses. | Comorbidities (inflammatory bowel disease, obesity, inherited thrombophilia); cancer (18-49, 50-64, and >65 y). | |||||||
| Treatments (CVC, mechanical ventilation, urinary catheter, chemotherapy, steroids) | |||||||||
| Bembenek et al35 (Poland) | Early stroke patients (N = 299) | 2007-2009 | 75 (64-82)* | Prognostic factor (single-center, prospective cohort) | Oral anticoagulation: 7.1% | Any symptomatic or asymptomatic DVT (n = 9; 7 of which were distal) | The first ultrasonography was performed within the first 7 d and then 8-10 d after stroke onset by a trained physician blinded to patients’ baseline health status, to identify patients in whom DVT occurred early in the course of stroke. | Days 3 and 9 after stroke | Age for each additional 10 y; female; hypertension; congestive heart failure; atrial fibrillation; diabetes; smoking status (current and previous); prestroke disability (mRS 0-1 patient and mRS 0-2 patients); stroke severity (each additional 4 patients. NIHSS, NIHSS >7 patients, NIHSS >14 patients); decreased consciousness (≥1 patient in NIHSS, ≥2 patients in NIHSS); inflammatory markers (CRP >10 mg/L, fibrinogen >4 mg/dL) |
| The model was not adjusted for oral anticoagulation, but less than 10% of the included patients received prophylaxis. | |||||||||
| Fan et al36 (China) | Acutely ill medical patients (N = 458) | 2006-2007 | 77 (7) | Prognostic factor (multicenter prospective cohort) | Pharmacological VTE prophylaxis: 0% | Any symptomatic or asymptomatic VTE (DVT or PE; n = 45: 30 symptomatic and 15 asymptomatic) | VTE verified by compression ultrasonography at enrollment and 3-wk follow-up. | 90 d follow-up for symptomatic and 3 wk for asymptomatic | Univariate model with results provided; a multivariate analysis was conducted but results of each factor were not reported. |
| Mechanical VTE prophylaxis (graduated compression stockings): 0.4% | Symptomatic cases were all screened by lower limb color duplex ultrasonography. | ||||||||
| The model was not adjusted for the mechanical thromboprophylaxis. | |||||||||
| Kelly et al38 (United Kingdom) | Acute ischemic stroke (N = 102) | Not reported | 70 (12) | Prognostic factor (single-center prospective cohort) | VTE prophylaxis: 0% | Any symptomatic or asymptomatic VTE (defined as proximal DVT or PE; n = 41). | Patients were assessed weekly for clinical evidence of VTE. New increases in calf circumference from initial assessment of ≥3 cm (based on the Wells scoring system), local pain or tenderness for DVT, and oxygen saturations ≤92% and/or respiratory rate >20/min in a patient otherwise asymptomatic for PE. | 21 d | Age >70; BI ≤9, total anterior circulation infarcts, malignancy, and atrial fibrillation |
| VTE was classified as “unrecognized clinical” if associated with the aforementioned signs or symptoms that went unrecognized by the attending team. | |||||||||
| Magnetic resonance direct thrombus imaging was performed. If DVT was identified, thoracic imaging was performed to detect PE. All scans were reviewed independently by 2 reviewers who reached a consensus. | |||||||||
| Clinical events diagnosed conventionally and data from postmortem examinations were included. | |||||||||
| Ota et al38 (Japan) | Congestive heart failure (N = 161) | 2003-2008 | 69.3 (10.8) | Prognostic factor (single-center, prospective cohort). | Anticoagulant therapy in DVT cases: 38.9%; non-DVT cases: 44.1% | Any symptomatic or asymptomatic DVT (no PE was detected; n = 18). | DVT verified by standardized sonography criterion of venous noncompressibility. | 11.8 ± 11.5 d | NYHA functional class; poor IVC collapsibility; no anticoagulation therapy |
| Antiplatelet therapy in DVT cases: 66.7% and in the non-DVT: 62.9% | PE verified by pulmonary angiography. | ||||||||
| The model was adjusted for anticoagulant therapy. | |||||||||
| Yi et al39 (China) | Acute stroke patients (N = 1380) | 2009-2010 | 69.8 (11.6) | Prognostic factor (multicenter, prospective cohort) | Pharmacological VTE prophylaxis with warfarin or LMWH: 15% | Any symptomatic or asymptomatic VTE (any PE and any DVT) (n = 62; 32 symptomatic DVT and 30 asymptomatic DVT) | DVT verified by VDU, venous angiography or venous CTA examination. | 12 mo | For PE as an outcome: |
| The model was adjusted for prophylaxis. | PE verified by chest CTA or pulmonary angiography. | Age ≥70 y, bedridden, incidence of DVT | |||||||
| For DVT as an outcome: | |||||||||
| Age ≥70 y; bedridden, Wells score ≥2, NIHSS score of lower limbs ≥3, BI score, rehabilitative therapy, anticoagulant therapy, concentration of D-dimer evaluated at admission. | |||||||||
| Bleeding | |||||||||
| Decousus et al8 (Canada) | Acutely ill medical patients (N = 15 156) | 2002-2006 | 68.2 (51.8-78.9)* | Prognostic model development (multicenter, prospective cohort) | Pharmacological VTE prophylaxis: 48% (LMWH, 38.4%; unfractionated heparin, 11.1%, aspirin, 0.7%) | Major or clinically relevant bleeding (n = 230; 83 major and 147 nonmajor, but clinically relevant, bleeding) | Major bleeding was defined as a bleeding event contributing to death, clinically overt bleeding associated with a decrease in hemoglobin level of ≥2 g/dL or leading to transfusion of at least 2 units of packed RBCs, or bleeding within a critical organ (including intracranial, retroperitoneal, intraocular, adrenal gland, spinal, or pericardial bleeding). | 14 d | Active gastroduodenal ulcer; bleeding in 3 mo before admission; platelet count <50 × 109/L; age ≥85 vs <40 y; hepatic failure; severe renal failure GFR <30 vs ≥60 mL/min/m2; ICU/CCU; CVC; rheumatic disease; current cancer; age 40-84 vs <40 y; male sex; moderate renal failure (GFR 30-59 vs ≥60 mL/min/m2). |
| Mechanical VTE prophylaxis: 9% (elastic stockings, 5.4%; intermittent pneumatic compression, 3.8%) | Nonmajor but clinically relevant bleeding was defined as overt gastrointestinal bleeding (except for insignificant hemorrhoidal bleeding), gross hematuria (macroscopic and lasting longer than 24 h), substantial epistaxis that required intervention and was recurrent and/or lasted at least 5 min, extensive hematoma or bruising (>5 cm in diameter), intra-articular bleeding (documented by aspiration), menorrhagia or metrorrhagia (increased quantity or duration), or other bleeding important enough to be recorded on the hospital chart. | ||||||||
| There were no differences in the estimates of associations when the model was adjusted for pharmacologic prophylaxis use. | |||||||||
| Mahan et al32 (US) | Medical patients (N = 327 578) | 2005-2009 | 69 | Prognostic factor (multicenter retrospective cohort) | All antithrombotic agent use: 9.4% | Major or clinically relevant bleeding (n = 29 264; 5 951 major and 23 313 minor bleeding) | Bleeding events were identified through the ICD-9-CM diagnosis codes. | Within 30 d after hospitalization | Age (40-54, 55-64, 65-74, ≥75); male; preindex risk factors (insufficient renal function, cancer, rheumatoid arthritis, gastroduodenal ulcer, blood dyscrasias, thrombocytopenia, liver disease, CVC, thromboembolic stroke, estrogen use); postindex risk factors (postdischarge antithrombotic meds use, rehospitalization, length of stay (2, 3-5, and ≥6 d) |
| Anticoagulants: 3.9% (warfarin, 3.6%; enoxaparin, 0.4%; heparin, 0.1% and other, <0.0%) | |||||||||
| Antiplatelets: 5.7% (clopidogrel: 4.6%; aspirin-dipyridamole, 0.9%; other, 0.3%) | |||||||||
| Anticoagulants and antiplatelets: 0.2% | |||||||||
| The model was adjusted for antithrombotic use. | |||||||||
| Patell et al33 (US) | Cancer patients (N = 3358) | 2012-2014 | 62(19-98)* | Prognostic factor (single- center retrospective cohort) | Antiplatelets: 14% | Major or clinically relevant bleeding (n = 69; 51 major and 18 nonmajor but clinically relevant bleeding) | Bleeding was assessed using the International Society on Thrombosis Hemostasis definitions of major bleeding and clinically relevant nonmajor bleeding | Median length of stay was 5 d (range, 0-152) days. | Reason for admission (anemia); BMI ≥40; cancer site: GI; low hemoglobin (<13 g/dL for men and <11.5 g/dL for women); low platelets (<150 000/μL) |
| Anticoagulants: 67% | Bleeding events were identified from discharge summaries of admissions being studied. To obtain details of event, documentation including diagnostic tests (imaging and procedures) and clinical notes was used. All bleeding events were confirmed manually by 2 investigators (third-year internal medicine residents at the time of the study). When unclear, individual cases were cross-reviewed, discussed and included if both agreed. No separate training was performed, and no coding was used to extract bleeding information. | ||||||||
| Antiplatelet agents on day of admission were not found to be statistically significant in univariate analysis, so were not added to the multivariate regression analysis mode. | |||||||||
| Anticoagulation exposure on admission was noted to be associated with a decreased risk of bleeding (OR, 0.5; 95% CI, 0.3-0.8; P = 0.004) although this was not significant in multivariable analysis (but the model adjusted for it). | |||||||||
| First author, year (country) . | Population (sample size) . | Time frame (y) . | Mean age, y (SD)* . | Study type (number of centers and study design) . | Prophylaxis (%) . | Outcome (number of events) . | Diagnostic methods . | Follow-up time . | Variables in multivariate logistic regression, when applicable . |
|---|---|---|---|---|---|---|---|---|---|
| Venous thromboembolism | |||||||||
| Spyropoulos et al7 (US) | Acutely ill medical patients (N = 15 156) | 2002-2006 | 68 (52-79)* | Prognostic model development (multicenter prospective cohort) | VTE prophylaxis (adjusted-dose warfarin, elastic stockings, LMWH, unfractionated heparin, intermittent pneumatic compression, and aspirin): 44% | Any symptomatic VTE (lower extremity DVT, and PE) (n = 184) | Clinically observed VTE | 92 d | IMPROVE RAM-related factors: |
| The model was adjusted for VTE prophylaxis. | DVT verified by positive venogram or compression ultrasonography. | Age >60 y; prior cancer, prior VTE, ICU/CCU stay, lower limb paralysis, immobility | |||||||
| PE verified by positive lung scan, pulmonary angiogram, or spiral CT scan. | Known thrombophilia | ||||||||
| Fatal PE was defined as PE diagnosed at autopsy or, in the absence of autopsy, when PE was considered the most likely cause of death. | |||||||||
| Mahan et al25 (Canada) | Acute medical patients (N = 417. VTE cases: n = 139; non-VTE controls: n = 278) | 2005-2011 | Cases: 68; controls: 65 | External validation (multicenter retrospective case-control) | VTE prophylaxis, 0% | Any symptomatic VTE (lower extremity DVT, and PE; n = 139) | PE, verified by a positive pulmonary angiogram, spiral computed tomography, or high-probability ventilation/perfusion scan or at autopsy. | 92 d | IMPROVE RAM-related factors: |
| Lower extremity DVT verified by positive compression ultrasonography, computed tomography, or magnetic resonance imaging, or at autopsy. | Previous VTE; known thrombophilia; lower limb paralysis; current cancer; immobilization ≥7; ICU/CCU stay; age >60 y | ||||||||
| Rosenberg et al26 (US) | Medical patients (N = 539: VTE cases: n = 135 and non-VTE controls: n = 404) | 2009-2013 | 67 | External validation (multicenter retrospective case-control) | Any prophylaxis in VTE cases: 49% (of those 44% pharmacological VTE and 5% mechanical) | Any symptomatic VTE (n = 135) | VTE events identified using ICD-9 codes | Within 90 d following the index admission | IMPROVE RAM-related factors: same as those in Spyropoulos et al7 |
| Any prophylaxis in non-VTE controls: 45% (of those 40% pharmacological VTE and 5% mechanical) | |||||||||
| The results were essentially unchanged when the cases and controls were stratified into groups that received VTE prophylaxis, including pharmacological prophylaxis during hospitalization and those that did not. | |||||||||
| Zakai et al27 (US) | Patients admitted to medical services (N = 900: VTE cases: 299; and non-VTE controls: 601) | 2002-2009 | cases: 6317 ; controls: 6615 | Prognostic model development (single-center retrospective case-control) | Pharmacological prophylaxis in VTE cases: 64.6% and in non-VTE controls: 62.2%; | Any symptomatic VTE (upper and lower extremity DVT and PE; n = 299) | VTE events identified using ICD-9 VTE discharge codes. | Discharge or transfer from medical service | Venous thrombosis prophylaxis (mechanical; pharmacologic); demographics (age, sex, BMI); medical history (myocardial infarction, COPD, diabetes, chronic kidney disease); conditions active on admission (fever, COPD, pneumonia, any infection). |
| mechanical prophylaxis in VTE cases: 31.8% and in non-VTE controls: 27.6%; | Codes confirmed by clinician review. | ||||||||
| the model was adjusted for both mechanical and pharmacological prophylaxis. | Records were reviewed by a research nurse and all hospital-acquired VTE cases and 20% of noncases were reviewed by a physician. | ||||||||
| Zakai et al28 (US) | Medical patients (N = 188: VTE cases: 65 and non-VTE controls: 123) | 2000-2002 | 68 | External validation (single-center retrospective case-control) | VTE prophylaxis (including warfarin, unfractionated heparin, low molecular weight heparin or intermittent pneumatic compression devices): in VTE cases: 59%; and non-VTE controls 47%. | Any symptomatic VTE (upper and lower extremity DVT and PE; n = 65) | VTE events identified using ICD-9 VTE discharge codes. | LOS case: 16 (10-28); controls: 6 (4-10) | Trauma last 3 mo; active cancer past year; admission fever; leg edema on admission; immobility >72 h; bacterial infection (cellulitis, pneumonia, sepsis, other); platelet count >350 × 109/L; use of VTE prophylaxis |
| The model was adjusted for prophylaxis. | |||||||||
| Zhou et al29 (China) | Medically ill patients (N = 1804: VTE cases: 902 and non-VTE controls: 902) | 2013-2016 | Cases: 6017 ; controls: 5717 | External validation (single-center retrospective Case-control) | Any prophylaxis: in VTE Cases: 4.1% and in non-VTE controls: 6.1% | Any symptomatic VTE (defined as DVT or PE; n = 902) | DVT verified by positive compression ultrasonography and/or contrast venography. | 6 mo after discharge | Caprini RAM factors* |
| VTE prophylaxis included any mechanical use (intermittent pneumatic compression devices or sole vein pump) or pharmacological use (unfractionated heparin, LMWH, warfarin, fondaparinux sodium, etc.) | PE verified by positive pulmonary angiogram, spiral computed tomography, or high probability ventilation/perfusion scanning or at autopsy. | Padua RAM factors† | |||||||
| The model was adjusted for VTE prophylaxis | |||||||||
| Barclay et al30 (US) | Chronic Liver disease (N = 1581) | 2008-2011 | 51(11) | Prognostic factor (single-center retrospective cohort) | Pharmacological VTE prophylaxis: 24.8% (Unfractionated heparin: 9.7%; low molecular weight heparin: 88.0%; or both: 2.3%) | Any symptomatic VTE (including DVT, PE or portal vein thrombosis-PVT) (n = 23) | VTE event identified in the medical record. | 4-7 d | VTE prophylaxis |
| The model was adjusted for pharmacological prophylaxis | VTE confirmed with radiologic testing. | Active malignancy | |||||||
| Trauma or surgery during hospitalization | |||||||||
| History of VTE | |||||||||
| Grant et al31 (US) | Hospitalized medical patients (N = 63 548) | 2011-2014 | 66 | External validation (multicenter retrospective cohort) | Pharmacological VTE prophylaxis: 60.9% | Any symptomatic VTE (defined as proximal upper- or proximal lower-extremity DVT and PE) (n = 670) | VTE was clinically suspected. | 90 d | Caprini RAM factors* |
| The model was adjusted for the pharmacological prophylaxis | VTE was Image confirmed. | ||||||||
| Majority of events were identified by medical record review, 44 (6.6%) of the events were confirmed via telephone follow-up. | |||||||||
| Rothberg et al34 (US) | Medical Patients (N = 46 503) | 2004-2005 | NR | Prognostic model development (multicenter retrospective cohort) | Pharmacological VTE prophylaxis: 30% | Any symptomatic VTE (n = 1 052) | VTE verified by lower extremity ultrasonography, venography, CT angiogram, ventilation/perfusion scan, or pulmonary angiogram) on hospital day 3 or later | 30 30 d | Any prophylaxis; female; length of stay ≥6 d; age (18-49; 50-64, and >65 y); primary diagnosis (pneumonia, COPD, stroke, congestive heart failure, urinary tract infection, respiratory failure, septicemia) |
| There was no difference in the model estimates for the factors when the model was adjusted for prophylaxis. | Secondary diagnosis of VTE provided using ICD-9 diagnoses. | Comorbidities (inflammatory bowel disease, obesity, inherited thrombophilia); cancer (18-49, 50-64, and >65 y). | |||||||
| Treatments (CVC, mechanical ventilation, urinary catheter, chemotherapy, steroids) | |||||||||
| Bembenek et al35 (Poland) | Early stroke patients (N = 299) | 2007-2009 | 75 (64-82)* | Prognostic factor (single-center, prospective cohort) | Oral anticoagulation: 7.1% | Any symptomatic or asymptomatic DVT (n = 9; 7 of which were distal) | The first ultrasonography was performed within the first 7 d and then 8-10 d after stroke onset by a trained physician blinded to patients’ baseline health status, to identify patients in whom DVT occurred early in the course of stroke. | Days 3 and 9 after stroke | Age for each additional 10 y; female; hypertension; congestive heart failure; atrial fibrillation; diabetes; smoking status (current and previous); prestroke disability (mRS 0-1 patient and mRS 0-2 patients); stroke severity (each additional 4 patients. NIHSS, NIHSS >7 patients, NIHSS >14 patients); decreased consciousness (≥1 patient in NIHSS, ≥2 patients in NIHSS); inflammatory markers (CRP >10 mg/L, fibrinogen >4 mg/dL) |
| The model was not adjusted for oral anticoagulation, but less than 10% of the included patients received prophylaxis. | |||||||||
| Fan et al36 (China) | Acutely ill medical patients (N = 458) | 2006-2007 | 77 (7) | Prognostic factor (multicenter prospective cohort) | Pharmacological VTE prophylaxis: 0% | Any symptomatic or asymptomatic VTE (DVT or PE; n = 45: 30 symptomatic and 15 asymptomatic) | VTE verified by compression ultrasonography at enrollment and 3-wk follow-up. | 90 d follow-up for symptomatic and 3 wk for asymptomatic | Univariate model with results provided; a multivariate analysis was conducted but results of each factor were not reported. |
| Mechanical VTE prophylaxis (graduated compression stockings): 0.4% | Symptomatic cases were all screened by lower limb color duplex ultrasonography. | ||||||||
| The model was not adjusted for the mechanical thromboprophylaxis. | |||||||||
| Kelly et al38 (United Kingdom) | Acute ischemic stroke (N = 102) | Not reported | 70 (12) | Prognostic factor (single-center prospective cohort) | VTE prophylaxis: 0% | Any symptomatic or asymptomatic VTE (defined as proximal DVT or PE; n = 41). | Patients were assessed weekly for clinical evidence of VTE. New increases in calf circumference from initial assessment of ≥3 cm (based on the Wells scoring system), local pain or tenderness for DVT, and oxygen saturations ≤92% and/or respiratory rate >20/min in a patient otherwise asymptomatic for PE. | 21 d | Age >70; BI ≤9, total anterior circulation infarcts, malignancy, and atrial fibrillation |
| VTE was classified as “unrecognized clinical” if associated with the aforementioned signs or symptoms that went unrecognized by the attending team. | |||||||||
| Magnetic resonance direct thrombus imaging was performed. If DVT was identified, thoracic imaging was performed to detect PE. All scans were reviewed independently by 2 reviewers who reached a consensus. | |||||||||
| Clinical events diagnosed conventionally and data from postmortem examinations were included. | |||||||||
| Ota et al38 (Japan) | Congestive heart failure (N = 161) | 2003-2008 | 69.3 (10.8) | Prognostic factor (single-center, prospective cohort). | Anticoagulant therapy in DVT cases: 38.9%; non-DVT cases: 44.1% | Any symptomatic or asymptomatic DVT (no PE was detected; n = 18). | DVT verified by standardized sonography criterion of venous noncompressibility. | 11.8 ± 11.5 d | NYHA functional class; poor IVC collapsibility; no anticoagulation therapy |
| Antiplatelet therapy in DVT cases: 66.7% and in the non-DVT: 62.9% | PE verified by pulmonary angiography. | ||||||||
| The model was adjusted for anticoagulant therapy. | |||||||||
| Yi et al39 (China) | Acute stroke patients (N = 1380) | 2009-2010 | 69.8 (11.6) | Prognostic factor (multicenter, prospective cohort) | Pharmacological VTE prophylaxis with warfarin or LMWH: 15% | Any symptomatic or asymptomatic VTE (any PE and any DVT) (n = 62; 32 symptomatic DVT and 30 asymptomatic DVT) | DVT verified by VDU, venous angiography or venous CTA examination. | 12 mo | For PE as an outcome: |
| The model was adjusted for prophylaxis. | PE verified by chest CTA or pulmonary angiography. | Age ≥70 y, bedridden, incidence of DVT | |||||||
| For DVT as an outcome: | |||||||||
| Age ≥70 y; bedridden, Wells score ≥2, NIHSS score of lower limbs ≥3, BI score, rehabilitative therapy, anticoagulant therapy, concentration of D-dimer evaluated at admission. | |||||||||
| Bleeding | |||||||||
| Decousus et al8 (Canada) | Acutely ill medical patients (N = 15 156) | 2002-2006 | 68.2 (51.8-78.9)* | Prognostic model development (multicenter, prospective cohort) | Pharmacological VTE prophylaxis: 48% (LMWH, 38.4%; unfractionated heparin, 11.1%, aspirin, 0.7%) | Major or clinically relevant bleeding (n = 230; 83 major and 147 nonmajor, but clinically relevant, bleeding) | Major bleeding was defined as a bleeding event contributing to death, clinically overt bleeding associated with a decrease in hemoglobin level of ≥2 g/dL or leading to transfusion of at least 2 units of packed RBCs, or bleeding within a critical organ (including intracranial, retroperitoneal, intraocular, adrenal gland, spinal, or pericardial bleeding). | 14 d | Active gastroduodenal ulcer; bleeding in 3 mo before admission; platelet count <50 × 109/L; age ≥85 vs <40 y; hepatic failure; severe renal failure GFR <30 vs ≥60 mL/min/m2; ICU/CCU; CVC; rheumatic disease; current cancer; age 40-84 vs <40 y; male sex; moderate renal failure (GFR 30-59 vs ≥60 mL/min/m2). |
| Mechanical VTE prophylaxis: 9% (elastic stockings, 5.4%; intermittent pneumatic compression, 3.8%) | Nonmajor but clinically relevant bleeding was defined as overt gastrointestinal bleeding (except for insignificant hemorrhoidal bleeding), gross hematuria (macroscopic and lasting longer than 24 h), substantial epistaxis that required intervention and was recurrent and/or lasted at least 5 min, extensive hematoma or bruising (>5 cm in diameter), intra-articular bleeding (documented by aspiration), menorrhagia or metrorrhagia (increased quantity or duration), or other bleeding important enough to be recorded on the hospital chart. | ||||||||
| There were no differences in the estimates of associations when the model was adjusted for pharmacologic prophylaxis use. | |||||||||
| Mahan et al32 (US) | Medical patients (N = 327 578) | 2005-2009 | 69 | Prognostic factor (multicenter retrospective cohort) | All antithrombotic agent use: 9.4% | Major or clinically relevant bleeding (n = 29 264; 5 951 major and 23 313 minor bleeding) | Bleeding events were identified through the ICD-9-CM diagnosis codes. | Within 30 d after hospitalization | Age (40-54, 55-64, 65-74, ≥75); male; preindex risk factors (insufficient renal function, cancer, rheumatoid arthritis, gastroduodenal ulcer, blood dyscrasias, thrombocytopenia, liver disease, CVC, thromboembolic stroke, estrogen use); postindex risk factors (postdischarge antithrombotic meds use, rehospitalization, length of stay (2, 3-5, and ≥6 d) |
| Anticoagulants: 3.9% (warfarin, 3.6%; enoxaparin, 0.4%; heparin, 0.1% and other, <0.0%) | |||||||||
| Antiplatelets: 5.7% (clopidogrel: 4.6%; aspirin-dipyridamole, 0.9%; other, 0.3%) | |||||||||
| Anticoagulants and antiplatelets: 0.2% | |||||||||
| The model was adjusted for antithrombotic use. | |||||||||
| Patell et al33 (US) | Cancer patients (N = 3358) | 2012-2014 | 62(19-98)* | Prognostic factor (single- center retrospective cohort) | Antiplatelets: 14% | Major or clinically relevant bleeding (n = 69; 51 major and 18 nonmajor but clinically relevant bleeding) | Bleeding was assessed using the International Society on Thrombosis Hemostasis definitions of major bleeding and clinically relevant nonmajor bleeding | Median length of stay was 5 d (range, 0-152) days. | Reason for admission (anemia); BMI ≥40; cancer site: GI; low hemoglobin (<13 g/dL for men and <11.5 g/dL for women); low platelets (<150 000/μL) |
| Anticoagulants: 67% | Bleeding events were identified from discharge summaries of admissions being studied. To obtain details of event, documentation including diagnostic tests (imaging and procedures) and clinical notes was used. All bleeding events were confirmed manually by 2 investigators (third-year internal medicine residents at the time of the study). When unclear, individual cases were cross-reviewed, discussed and included if both agreed. No separate training was performed, and no coding was used to extract bleeding information. | ||||||||
| Antiplatelet agents on day of admission were not found to be statistically significant in univariate analysis, so were not added to the multivariate regression analysis mode. | |||||||||
| Anticoagulation exposure on admission was noted to be associated with a decreased risk of bleeding (OR, 0.5; 95% CI, 0.3-0.8; P = 0.004) although this was not significant in multivariable analysis (but the model adjusted for it). | |||||||||
BI, Barthel Index; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CTA, computed tomographic angiography; GI, gastrointestinal; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IVC, inferior vena cava; LWMH, low-molecular-weight heparin; mRS, modified Rankin Score; NYHA, New York Heart Association; RBC, red blood cell.
Caprini factors: stroke; acute spinal cord injury or paralysis (<1 mo); hip, pelvis, or leg fracture (<1 mo); multiple trauma (<1 mo); age ≥75); history of VTE; family history of VTE; history of thrombophilia; heparin-induced thrombocytopenia; age (41-60); age (61-74); positive history of cancer; immobilizing plaster cast; congestive heart failure; COPD or pulmonary function; inflammatory bowel disease; severe long disease (including pneumonia); acute myocardial infarction; sepsis (<1 mo); surgery (<1 mo); postpartum (<1 mo); history of unexpected stillborn infant, recurrent spontaneous abortion (≥ 3) or premature birth; varicose veins; BMI >25 kg/m2; swollen legs (current); CVC present on admission; immobile or not ambulating; and hormone replacement therapy or oral contraceptives.
Padua factors: active cancer; previous VTE; reduced mobility; known thrombophilia; recent trauma and /or surgery; elderly age; heart and/or respiratory failure; acute myocardial infarction or ischemic stroke; acute infection and/or rheumatologic disorder; obesity; ongoing hormone treatment, and VTE prophylaxis.
Risk-of-bias assessment
Risk of bias was serious across all identified studies, each presenting risk of bias in at least 1 domain or item (Tables 2 and 3). Among the 17 included studies, 10 were retrospective, which may have introduced classification bias.25-34 Seven of the 8 prognostic factor studies included only the variables significant in bivariable analysis in their final regression model and did not present any data for nonsignificant predictors in their adjusted analysis.30,32,33,36-39 Two of the 8 prognostic factor studies32,33 and 4 of the 9 prognostic model development or validation studies did not have a clear description of appropriate outcome measurement.26-29 We detected no evidence of publication bias through visual assessment of asymmetry of the funnel plot for each pooled predictor in those that included at least 10 studies (Tables 2 and 3). Supplemental Table 2 provides the detailed judgements for each of the risk-of-bias domain criteria.
Evidence profile for VTE-related prognostic factors
| No. of studies . | Certainty assessment domains . | Overall certainty in the evidence about this prognostic factor . | Relative effect (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| Study design . | Risk of bias . | Indirect . | Inconsistent . | Imprecise . | Publication bias . | |||
| Age (>60 y vs <60 y)7,25-27,29,31,34,37,39 | ||||||||
| 9* | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.34 (95% CI, 1.17-1.55) |
| MODERATE | ||||||||
| Sex (male vs female)27,34-37 | ||||||||
| 5 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.03 (95% CI, 0.80-1.33) |
| MODERATE | ||||||||
| CRP (>10 mg/L vs <10 mg/L)35 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 10.10 (95% CI, 1.93-52.85) |
| MODERATE | ||||||||
| D-dimer (>500 ng/mL vs <500 ng/mL at baseline; and increase vs no increase)36,39 | ||||||||
| 2 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Categorical: OR, 2.46 (95% CI, 1.19-5.10) |
| MODERATE | Continuous: OR, 3.45 (95% CI, 2.01-5.92) | |||||||
| Heart rate (elevated >100 beats per minute vs nonelevated <100 beats per minute)27 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.48 (95% CI, 1.66-3.71) |
| MODERATE | ||||||||
| Thrombocytosis (platelet count >350 × 109/L vs <350 × 109/L)27,28 | ||||||||
| 2 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.16 (95% CI, 1.40-3.35) |
| MODERATE | ||||||||
| Leukocytosis (WBC ≥11 × 109/L vs <11 × 109/L)27 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.91 (95% CI, 1.24-2.94) |
| MODERATE | ||||||||
| Fever (body temperature >38°C-38.5°C vs <38°C-38.5°C)27,28 | ||||||||
| 2 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.88 (95% CI, 1.10-3.21) |
| MODERATE | ||||||||
| Leg edema (presence vs absence)28,31 | ||||||||
| 2 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.88 (95% CI, 1.23-2.90) |
| MODERATE | ||||||||
| Varicose veins (presence vs absence)29,31 | ||||||||
| 2 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | OR, 1.53 (95% CI, 0.85-2.76) |
| LOW | ||||||||
| Obesity (BMI >30 kg/m2vs <30 kg/m2)27,29,34 | ||||||||
| 3 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | OR, 1.34 (95% CI, 0.94-1.91) |
| LOW | ||||||||
| Fibrinogen levels (elevated levels >400 mg/dL) vs no elevated levels35 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 0.18 (95% CI, 0.04-0.81) |
| MODERATE | ||||||||
| BI score ≤9 vs >937,39 | ||||||||
| 2 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 8.30 (95% CI, 2.70-25.52) |
| MODERATE | ||||||||
| Immobility: defined as confinement to bed for >72 h or >7 d or bedridden or nonambulatory (yes vs no)7,26-29,31,37,39 | ||||||||
| 8 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 3.17 (95% CI, 2.18-4.62) |
| MODERATE | ||||||||
| Paresis (yes vs no)7,25,26,37 | ||||||||
| 4 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.97 (95% CI, 1.20-7.36) |
| MODERATE | ||||||||
| Previous VTE (yes vs no)7,25-27,29-31,36 | ||||||||
| 8 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 6.08 (95% CI, 3.71-9.97). |
| MODERATE | ||||||||
| Thrombophilia (familial or acquired disorder of the hemostatic system; yes vs no)7,25,26,29,34 | ||||||||
| 5 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 5.88 (95% CI, 2.80-12.35) |
| MODERATE | ||||||||
| Malignancy (active malignancy, defined as the presence of cancer on admission or within the past year, vs no active malignancy; and history vs no history of malignancy)7,25-28,30,31,34,36,37 | ||||||||
| 10 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Active cancer: OR, 2.65 (95% CI, 1.79-3.91) |
| MODERATE | History of cancer: OR, 3.20 (95% CI, 2.14-4.79) | |||||||
| Critical illness: defined as ICU or CCU stay, or need for resuscitation (yes vs no)7,25-27,29,34,36 | ||||||||
| 7 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.65 (95% CI, 1.39-1.95) |
| MODERATE | ||||||||
| Infections: including cellulitis, pneumonia, and sepsis (yes vs no)28,31 | ||||||||
| 5 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Any infection: OR, 1.48 (95% CI, 1.16-1.89) |
| MODERATE | Acute infection: OR, 1.59 (95% CI, 1.23-2.06) | |||||||
| Sepsis: OR, 1.07 (95% CI, 0.70-1.62) | ||||||||
| Heart failure (HF) (acute HF vs no acute HF; history of HF vs no history of HF)28,29,31,35,36 | ||||||||
| 5 | Observational | Serious | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Acute heart failure: OR, 0.82 (95% CI, 0.42-1.60) |
| LOW | History of heart failure: OR, 2.68 (95% CI, 1.11-6.44) | |||||||
| Autoimmune disease: including rheumatological diseases and inflammatory diseases (yes vs no)27,29,31,35 | ||||||||
| 4 | Observational | Serious† | Not serious | Serious | Not serious | Undetected | ⊕⊕○○ | OR, 2.33 (95% CI, 1.13-4.83) |
| LOW | ||||||||
| CVC (presence vs absence)31,34 | ||||||||
| 2 | Observational | Serious | Not serious | Serious§ | Not serious | Undetected | ⊕⊕○○ | OR, 2.05 (95% CI, 0.74-5.65) |
| LOW | ||||||||
| Severe stroke: defined as acute ischemic stroke (yes vs no)29,31,32,36 | ||||||||
| 4 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Acute ischemic stroke: OR, 1.79 (95% CI, 0.77-4.18) |
| LOW | When stroke was assessed in terms of the NIHSS, we found consistent results.¶ | |||||||
| Tobacco (current use vs no current use; previous use vs no previous use)35 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Current tobacco use: OR, 1.59 (95% CI, 0.28-9.03) |
| LOW | Previous tobacco use: OR, 0.97 (0.24-3.92) | |||||||
| Hormone use: defined as estrogen intake (yes vs no)31 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | OR, 0.80 (95% CI, 0.36-1.78). |
| LOW | ||||||||
| Renal failure(yes vs no)36 | ||||||||
| 1 | Observational | Serious || | Not serious | Not serious | Very Serious‡,# | Undetected | ⊕○○○ | OR, 0.76 (95% CI, 0.18-3.18) |
| VERY LOW | ||||||||
| Respiratory failure (yes vs no)27,29,31,36 | ||||||||
| 4 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Any respiratory failure: OR, 1.04 (95% 0.69-1.58). |
| LOW | Acute respiratory failure: OR, 1.18 (95% CI, 0.76-1.84) | |||||||
| Chronic respiratory failure: OR, 0.58 (95% 0.30-1.10) | ||||||||
| Coronary artery disease (yes vs no)29,31,36,37 | ||||||||
| 4 | Observational | Serious† | Not serious | Serious§ | Not serious | Undetected | ⊕⊕○○ | OR, 1.01 (95% CI, 0.33-3.09) |
| LOW | ||||||||
| No. of studies . | Certainty assessment domains . | Overall certainty in the evidence about this prognostic factor . | Relative effect (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| Study design . | Risk of bias . | Indirect . | Inconsistent . | Imprecise . | Publication bias . | |||
| Age (>60 y vs <60 y)7,25-27,29,31,34,37,39 | ||||||||
| 9* | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.34 (95% CI, 1.17-1.55) |
| MODERATE | ||||||||
| Sex (male vs female)27,34-37 | ||||||||
| 5 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.03 (95% CI, 0.80-1.33) |
| MODERATE | ||||||||
| CRP (>10 mg/L vs <10 mg/L)35 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 10.10 (95% CI, 1.93-52.85) |
| MODERATE | ||||||||
| D-dimer (>500 ng/mL vs <500 ng/mL at baseline; and increase vs no increase)36,39 | ||||||||
| 2 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Categorical: OR, 2.46 (95% CI, 1.19-5.10) |
| MODERATE | Continuous: OR, 3.45 (95% CI, 2.01-5.92) | |||||||
| Heart rate (elevated >100 beats per minute vs nonelevated <100 beats per minute)27 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.48 (95% CI, 1.66-3.71) |
| MODERATE | ||||||||
| Thrombocytosis (platelet count >350 × 109/L vs <350 × 109/L)27,28 | ||||||||
| 2 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.16 (95% CI, 1.40-3.35) |
| MODERATE | ||||||||
| Leukocytosis (WBC ≥11 × 109/L vs <11 × 109/L)27 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.91 (95% CI, 1.24-2.94) |
| MODERATE | ||||||||
| Fever (body temperature >38°C-38.5°C vs <38°C-38.5°C)27,28 | ||||||||
| 2 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.88 (95% CI, 1.10-3.21) |
| MODERATE | ||||||||
| Leg edema (presence vs absence)28,31 | ||||||||
| 2 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.88 (95% CI, 1.23-2.90) |
| MODERATE | ||||||||
| Varicose veins (presence vs absence)29,31 | ||||||||
| 2 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | OR, 1.53 (95% CI, 0.85-2.76) |
| LOW | ||||||||
| Obesity (BMI >30 kg/m2vs <30 kg/m2)27,29,34 | ||||||||
| 3 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | OR, 1.34 (95% CI, 0.94-1.91) |
| LOW | ||||||||
| Fibrinogen levels (elevated levels >400 mg/dL) vs no elevated levels35 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 0.18 (95% CI, 0.04-0.81) |
| MODERATE | ||||||||
| BI score ≤9 vs >937,39 | ||||||||
| 2 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 8.30 (95% CI, 2.70-25.52) |
| MODERATE | ||||||||
| Immobility: defined as confinement to bed for >72 h or >7 d or bedridden or nonambulatory (yes vs no)7,26-29,31,37,39 | ||||||||
| 8 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 3.17 (95% CI, 2.18-4.62) |
| MODERATE | ||||||||
| Paresis (yes vs no)7,25,26,37 | ||||||||
| 4 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.97 (95% CI, 1.20-7.36) |
| MODERATE | ||||||||
| Previous VTE (yes vs no)7,25-27,29-31,36 | ||||||||
| 8 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 6.08 (95% CI, 3.71-9.97). |
| MODERATE | ||||||||
| Thrombophilia (familial or acquired disorder of the hemostatic system; yes vs no)7,25,26,29,34 | ||||||||
| 5 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 5.88 (95% CI, 2.80-12.35) |
| MODERATE | ||||||||
| Malignancy (active malignancy, defined as the presence of cancer on admission or within the past year, vs no active malignancy; and history vs no history of malignancy)7,25-28,30,31,34,36,37 | ||||||||
| 10 | Observational | Serious | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Active cancer: OR, 2.65 (95% CI, 1.79-3.91) |
| MODERATE | History of cancer: OR, 3.20 (95% CI, 2.14-4.79) | |||||||
| Critical illness: defined as ICU or CCU stay, or need for resuscitation (yes vs no)7,25-27,29,34,36 | ||||||||
| 7 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.65 (95% CI, 1.39-1.95) |
| MODERATE | ||||||||
| Infections: including cellulitis, pneumonia, and sepsis (yes vs no)28,31 | ||||||||
| 5 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Any infection: OR, 1.48 (95% CI, 1.16-1.89) |
| MODERATE | Acute infection: OR, 1.59 (95% CI, 1.23-2.06) | |||||||
| Sepsis: OR, 1.07 (95% CI, 0.70-1.62) | ||||||||
| Heart failure (HF) (acute HF vs no acute HF; history of HF vs no history of HF)28,29,31,35,36 | ||||||||
| 5 | Observational | Serious | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Acute heart failure: OR, 0.82 (95% CI, 0.42-1.60) |
| LOW | History of heart failure: OR, 2.68 (95% CI, 1.11-6.44) | |||||||
| Autoimmune disease: including rheumatological diseases and inflammatory diseases (yes vs no)27,29,31,35 | ||||||||
| 4 | Observational | Serious† | Not serious | Serious | Not serious | Undetected | ⊕⊕○○ | OR, 2.33 (95% CI, 1.13-4.83) |
| LOW | ||||||||
| CVC (presence vs absence)31,34 | ||||||||
| 2 | Observational | Serious | Not serious | Serious§ | Not serious | Undetected | ⊕⊕○○ | OR, 2.05 (95% CI, 0.74-5.65) |
| LOW | ||||||||
| Severe stroke: defined as acute ischemic stroke (yes vs no)29,31,32,36 | ||||||||
| 4 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Acute ischemic stroke: OR, 1.79 (95% CI, 0.77-4.18) |
| LOW | When stroke was assessed in terms of the NIHSS, we found consistent results.¶ | |||||||
| Tobacco (current use vs no current use; previous use vs no previous use)35 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Current tobacco use: OR, 1.59 (95% CI, 0.28-9.03) |
| LOW | Previous tobacco use: OR, 0.97 (0.24-3.92) | |||||||
| Hormone use: defined as estrogen intake (yes vs no)31 | ||||||||
| 1 | Observational | Serious | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | OR, 0.80 (95% CI, 0.36-1.78). |
| LOW | ||||||||
| Renal failure(yes vs no)36 | ||||||||
| 1 | Observational | Serious || | Not serious | Not serious | Very Serious‡,# | Undetected | ⊕○○○ | OR, 0.76 (95% CI, 0.18-3.18) |
| VERY LOW | ||||||||
| Respiratory failure (yes vs no)27,29,31,36 | ||||||||
| 4 | Observational | Serious† | Not serious | Not serious | Serious‡ | Undetected | ⊕⊕○○ | Any respiratory failure: OR, 1.04 (95% 0.69-1.58). |
| LOW | Acute respiratory failure: OR, 1.18 (95% CI, 0.76-1.84) | |||||||
| Chronic respiratory failure: OR, 0.58 (95% 0.30-1.10) | ||||||||
| Coronary artery disease (yes vs no)29,31,36,37 | ||||||||
| 4 | Observational | Serious† | Not serious | Serious§ | Not serious | Undetected | ⊕⊕○○ | OR, 1.01 (95% CI, 0.33-3.09) |
| LOW | ||||||||
Question: prognostic factors for medical patients; outcome: VTE; setting: inpatient.
BI, Barthel Index; WBC, white blood cell count.
Certainty in evidence was downgraded for risk of bias, given a follow-up time of more than 3 months in the included studies that may cause an overestimation of the magnitude of the association (Zhou et al29 : 6 mo after discharge and Yi 2012: 12 mo after discharge).
Certainty in evidence was downgraded for imprecision, given that the confidence interval suggests that there may be no association.
Certainty in evidence was downgraded for risk of bias, given that the results of each prognostic factor in the multivariate analysis were not reported and we therefore had to rely on the unadjusted measures of association. Also, the multivariate analysis included only factors statistically significant in the univariate analysis.
Certainty in evidence was downgraded for inconsistency but not imprecision, given that the inconsistency is the likely cause for the imprecision.
Certainty in evidence was downgraded for imprecision, given the small number of events (n = 32).
Bembenek et al35 assessed the severity of a stroke experienced by an individual by using the NIHSS, a diagnostic tool; results were consistent with the meta-analysis that severe stroke may result in an increase in risk of any DVT (OR, 2.11; 95% CI,: 0.50-8.90) for NIHSS >7 compared with a NIHSS score <7. Also, severe stroke may result in an increase in risk of any DVT (OR, 1.34; 95% CI,: 0.25-7.18) for NIHSS >14 compared with a NIHSS <14. When NIHSS was assessed continuously, results showed that severe stroke may result in an increase in risk of any DVT (OR, 1.21; 95% CI,: 0.86-1.70) for each additional 4 points on the NIHSS.
Fan et al,36 with 458 patients older than 60 y of which 45 patients had any VTE, presented age as a continuous variable and showed no association between age and any VTE (OR, 1.03; 95% CI, 0.98-1.08). Bembenek et al,35 with 299 patients of which 9 had any DVT, 7 of which were distal, presented age per 10-y increase and showed a decrease in risk per 10-y increase in age with any DVT (OR, 0.64; 95% CI, 0.33-1.24).
Evidence profile for bleeding-related prognostic factors
| No. of studies . | Certainty assessment domains . | Overall certainty in the evidence about this prognostic factor . | Relative effect (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| Study design . | Risk of bias . | Indirect . | Inconsistent . | Imprecise . | Publication bias . | |||
| Age (≥65 vs <65)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Age ≥65: OR, 1.95 (95% CI, 1.59-2.38) |
| MODERATE | ||||||||
| Sex (male vs female)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.27 (95% CI, 1.09-1.47). |
| MODERATE | ||||||||
| Anemia as a reason for admission (presence vs absence)34 | ||||||||
| 2 | Observational | Serious*,‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 5.15 (95% CI, 2.45-10.81) |
| MODERATE | ||||||||
| Morbid obesity (BMI ≥ 40 kg/m2 vs BMI < <40 kg/m2)34 | ||||||||
| 1 | Observational | Serious‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 3.08 (95% CI, 1.35-7.02) |
| MODERATE | ||||||||
| Low hemoglobin: defined as <13 g/dL in men and <11.5 g/dL in women (yes vs no)34 | ||||||||
| 1 | Observational | Serious‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.33 (95% CI, 1.04-5.22) |
| MODERATE | ||||||||
| Gastroduodenal ulcers (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.74 (95% CI, 1.42-5.26) |
| MODERATE | ||||||||
| Rehospitalization (yes vs no)33 | ||||||||
| 1 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.39 (95% 2.25-2.54) |
| MODERATE | ||||||||
| Critical illness (yes vs no)8 | ||||||||
| 1 | Observational | Serious* | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.10 (95% CI, 1.42-3.11). |
| MODERATE | ||||||||
| Thrombocytopenia (yes vs no)8,33,34 | ||||||||
| 3 | Observational | Serious*,†,‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | All: OR, 1.79 (95% CI, 0.97-3.29) |
| MODERATE | <50 × 109/L: OR, 3.37 (95% CI, 1.84-6.18) | |||||||
| <150 × 109/L: OR, 1.30 (95% CI, 0.92-1.82) | ||||||||
| Blood dyscrasias defined as the presence of any bleeding disorders on admission (presence vs absence)33 | ||||||||
| 1 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.70 (95% CI, 1.60-1.81) |
| MODERATE | ||||||||
| Hepatic disease (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.53 (95% CI, 1.09-2.15) |
| MODERATE | ||||||||
| Renal failure (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Total: OR, 1.43 (95% CI, 1.06-1.93) |
| MODERATE | Any renal failure (RF): OR, 1.23 (95% CI, 0.92-1.65). | |||||||
| Moderate RF (GFR 30-59 mL/min/m2): OR, 1.37(95% CI, 0.84-2.23) | ||||||||
| Severe RF (GFR <30 mL/min/m2): OR, 2.14 (95% CI, 1.22-3.75) | ||||||||
| Antithrombotic medication (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.28 (95% CI, 1.01-1.64) |
| MODERATE | ||||||||
| CVC (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.37 (95% CI, 0.83-2.26) |
| MODERATE | ||||||||
| Autoimmune disease (yes vs no)8 | ||||||||
| 2 | Observational | Serious* | Not serious | Not serious | Serious§ | Undetected | ⊕⊕○○ | OR, 1.30 (95% CI, 0.77-2.19) |
| LOW | ||||||||
| Hormone use: defined as estrogen intake (yes vs no)33 | ||||||||
| 1 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 0.95 (95% CI, 0.82-1.10) |
| MODERATE | ||||||||
| Malignancy (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious *,† | Not serious | Not serious | Serious§ | Undetected | ⊕⊕○○ | OR, 1.08 (95% CI, 0.42-2.77) |
| LOW | ||||||||
| No. of studies . | Certainty assessment domains . | Overall certainty in the evidence about this prognostic factor . | Relative effect (95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| Study design . | Risk of bias . | Indirect . | Inconsistent . | Imprecise . | Publication bias . | |||
| Age (≥65 vs <65)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Age ≥65: OR, 1.95 (95% CI, 1.59-2.38) |
| MODERATE | ||||||||
| Sex (male vs female)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.27 (95% CI, 1.09-1.47). |
| MODERATE | ||||||||
| Anemia as a reason for admission (presence vs absence)34 | ||||||||
| 2 | Observational | Serious*,‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 5.15 (95% CI, 2.45-10.81) |
| MODERATE | ||||||||
| Morbid obesity (BMI ≥ 40 kg/m2 vs BMI < <40 kg/m2)34 | ||||||||
| 1 | Observational | Serious‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 3.08 (95% CI, 1.35-7.02) |
| MODERATE | ||||||||
| Low hemoglobin: defined as <13 g/dL in men and <11.5 g/dL in women (yes vs no)34 | ||||||||
| 1 | Observational | Serious‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.33 (95% CI, 1.04-5.22) |
| MODERATE | ||||||||
| Gastroduodenal ulcers (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.74 (95% CI, 1.42-5.26) |
| MODERATE | ||||||||
| Rehospitalization (yes vs no)33 | ||||||||
| 1 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.39 (95% 2.25-2.54) |
| MODERATE | ||||||||
| Critical illness (yes vs no)8 | ||||||||
| 1 | Observational | Serious* | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 2.10 (95% CI, 1.42-3.11). |
| MODERATE | ||||||||
| Thrombocytopenia (yes vs no)8,33,34 | ||||||||
| 3 | Observational | Serious*,†,‡ | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | All: OR, 1.79 (95% CI, 0.97-3.29) |
| MODERATE | <50 × 109/L: OR, 3.37 (95% CI, 1.84-6.18) | |||||||
| <150 × 109/L: OR, 1.30 (95% CI, 0.92-1.82) | ||||||||
| Blood dyscrasias defined as the presence of any bleeding disorders on admission (presence vs absence)33 | ||||||||
| 1 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.70 (95% CI, 1.60-1.81) |
| MODERATE | ||||||||
| Hepatic disease (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.53 (95% CI, 1.09-2.15) |
| MODERATE | ||||||||
| Renal failure (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | Total: OR, 1.43 (95% CI, 1.06-1.93) |
| MODERATE | Any renal failure (RF): OR, 1.23 (95% CI, 0.92-1.65). | |||||||
| Moderate RF (GFR 30-59 mL/min/m2): OR, 1.37(95% CI, 0.84-2.23) | ||||||||
| Severe RF (GFR <30 mL/min/m2): OR, 2.14 (95% CI, 1.22-3.75) | ||||||||
| Antithrombotic medication (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.28 (95% CI, 1.01-1.64) |
| MODERATE | ||||||||
| CVC (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious*,† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 1.37 (95% CI, 0.83-2.26) |
| MODERATE | ||||||||
| Autoimmune disease (yes vs no)8 | ||||||||
| 2 | Observational | Serious* | Not serious | Not serious | Serious§ | Undetected | ⊕⊕○○ | OR, 1.30 (95% CI, 0.77-2.19) |
| LOW | ||||||||
| Hormone use: defined as estrogen intake (yes vs no)33 | ||||||||
| 1 | Observational | Serious† | Not serious | Not serious | Not serious | Undetected | ⊕⊕⊕○ | OR, 0.95 (95% CI, 0.82-1.10) |
| MODERATE | ||||||||
| Malignancy (yes vs no)8,33 | ||||||||
| 2 | Observational | Serious *,† | Not serious | Not serious | Serious§ | Undetected | ⊕⊕○○ | OR, 1.08 (95% CI, 0.42-2.77) |
| LOW | ||||||||
Question: prognostic factors for medical patients; outcome: bleeding; setting: inpatient.
Certainty in evidence was downgraded for risk of bias, given that patients were enrolled both prospectively and retrospectively in Decousus et al.8 The retrospective enrollment of patients may have introduced classification bias.
Certainty in evidence was downgraded for risk of bias, given that the authors evaluated bleeding risk in medical patients after hospitalization, that may overestimate the magnitude of the association. This is possibly due to patients being discharged on thromboprophylaxis without proper risk stratification for bleeding placing unmonitored patients at a higher risk of having a bleeding event.
Certainty in evidence was downgraded for risk of bias, given that the population is specific to hospitalized cancer patients who are at a higher risk of VTE and may be given thromboprophylaxis, placing them at a higher risk of having a bleeding event. This in turn may overestimate the magnitude of the association.
Certainty in evidence was downgraded for imprecision, given that the CI suggests that there may be no association.
Prognostic factors for VTE in hospitalized medical patients
Investigated were 29 candidate prognostic factors for VTE from 14 studies including 15 1 714 patients. Table 2 provides the evidence profile for VTE-related prognostic factors. Supplemental Figure 1 provides the forest plots of the meta-analysis of each of the prognostic factors.
Demographic factors
We found moderate-certainty evidence that there is probably an association between risk of any VTE and age ≥60 y (OR, 1.34; 95% CI, 1.17-1.55),7,25-27,29,31,34-37,39 and that there is probably little to no association between risk of any VTE and sex (males vs females: OR, 1.03; 95% CI, 0.80-1.33).27,34-37
Functional factors
There was moderate-certainty evidence for a probable association between risk of any VTE and lower Barthel Index scores (BI ≤ 9; OR, 8.30; 95% CI, 2.70-25.52)37 ; immobility, defined as confinement to bed for >72 hours or >7 days or bedridden or nonambulatory (OR, 3.17; 95% CI, 2.18-4.62)7,25,26,28,29,31,37,39 ; and paresis (OR, 2.97; 95% CI, 1.20-7.36).7,25,26,37
Medical illness and patient history factors
We identified moderate-certainty evidence for an association between risk of any VTE and history of VTE (OR, 6.08; 95% CI, 3.71-9.97)7,25-27,29-31,36 ; thrombophilia, defined as familial or acquired disorder of the hemostatic system (OR, 5.88; 95% CI, 2.80-12.35)7,25,26,29,34 ; history of malignancy (OR, 3.20; 95% CI, 2.14-4.79)27 ; active malignancy defined as the presence of cancer on admission or within the past year (OR, 2.65; 95% CI, 1.79-3.91)7,25,27,28,30,31,34,36,37 ; critical illness, defined as intensive care unit (ICU) or coronary care unit (CCU) stay or need for resuscitation (OR, 1.65; 95% CI, 1.39-1.95)7,25-27,29,34,36 ; and infections including cellulitis, pneumonia, and sepsis (OR, 1.48; 95% CI, 1.16-1.89).27-29,31,36
We found low-certainty evidence that there may be an association between risk of any VTE and history of heart failure (OR, of 2.68; 95% CI, 1.11-6.44)27,29,35 ; autoimmune diseases, including rheumatological diseases and inflammatory diseases (OR, 2.33; 95% CI, 1.13-4.83)31,34,36,39 ; central venous catheter (CVC) use (OR, 2.05; 95% CI, 0.74-5.65)31,34 ; and severe stroke, defined as acute ischemic stroke (OR, 1.79; 95% CI, 0.77-4.18).29,31,32,35,36 The findings for severe stroke when assessed using a diagnostic tool, the National Institutes of Health Stroke Scale (NIHSS), were consistent with our results (Table 2).35 We also identified low-certainty evidence that there may be an association between risk of any VTE and current tobacco use (OR, 1.59; 95% CI, 0.28-9.03); however, there may be little to no association between risk of any VTE and previous tobacco use (OR, 0.97; 95% CI, 0.24-3.92).
Furthermore, we identified low-certainty evidence that there may be little to no association between the risk of any VTE and respiratory failure (OR, 1.04; 95% CI, 0.69-1.58), coronary artery disease (CAD; OR, 1.01; 95% CI, 0.33-3.09), acute heart failure (OR, 0.82; 95% CI, 0.42-1.60),31,36 and hormone use (OR, 0.8; 95% CI, 0.36-1.78).29,31,36,37
We found very-low-certainty evidence that there may be little to no association between risk of any VTE and chronic renal failure (OR, 0.76; 95% CI, 0.18-3.18).36
Laboratory and physical examination factors
There is moderate-certainty evidence of an association between risk of any VTE and C-reactive protein (CRP) >10 mg/L (OR, 10.10; 95% CI, 1.93-52.85)35 and D-dimer >500 ng/mL at baseline (OR, 2.46; 95% CI, 1.19-5.10).36 The findings for D-dimer concentration, when assessed as a continuous variable, were consistent with our results (Table 2).39 Also, there is probably an association between risk of any VTE and elevated heart rate (>100 beats per minute; OR, 2.48; 95% CI, 1.66-3.71),27 thrombocytosis (platelet count, >350 × 109/L; OR, 2.16; 95% CI, 1.40-3.35),27,28 leukocytosis (white blood cell count, ≥11 × 109/L; OR, 1.91; 95% CI: 1.24-2.94),27 fever (body temperature >38°C-38.5°C; OR, 1.88; 95% CI, 1.10-3.21),27,28 leg edema (OR, 1.88; 95% CI, 1.23-2.90),28,31 and elevated fibrinogen level (>400 mg/dL; OR, 0.18; 95% CI, 0.04-0.81).35
We identified low-certainty evidence that there may be an association between risk of any VTE and varicose veins (OR, 1.53; 95% CI, 0.85-2.76)29,31 and obesity (BMI >30 kg/m2; OR, 1.34; 95% CI, 0.94-1.91).27,29,31,34,36
Additional analyses
We performed a sensitivity analysis, including studies that reported on immobility, to compare the association between immobility >72 hours and >7 days with risk of VTE. We found similar effect estimates for both categories with a slightly stronger association between immobility, defined as bed rest for >7 days, and risk of VTE (OR, 3.67; 95% CI, 0.85-15.93) compared with immobility, defined as bed rest for >72 hours, and risk of VTE (OR, 3.18; 95% CI, 1.10-9.16).
We also conducted a sensitivity analysis, including studies that reported on symptomatic VTE only, to evaluate the influence of the studies that reported on both symptomatic and asymptomatic VTE. The results of the sensitivity analysis showed similar effect estimates across prognostic factors except for CAD. The association between CAD and risk of symptomatic VTE was somewhat stronger in the sensitivity analysis of the 9 studies (OR, 2.02; 95% CI, 0.32-12.64), compared with little to no association with risk of VTE in the primary analysis (OR, 1.01; 95% CI, 0.33-3.09; supplemental Table 4).
Prognostic factors for bleeding in medical hospitalized patients
Three studies including 160 142 patients investigated 17 candidate prognostic factors for bleeding. Table 3 provides the evidence profile for bleeding-related prognostic factors. Supplemental Figure 2 provides the forest plots of the meta-analyses of each of the prognostic factors.
Demographic factors
Medical illness and patient history factors
There was moderate-certainty evidence of a probable association between risk of bleeding and gastroduodenal ulcers (OR, 2.74; 95% CI, 1.42-5.26),8,32 rehospitalization (OR, 2.39; 95% CI, 2.25- 2.54),32 critical illness including ICU or CCU stay (OR, 2.10; 95% CI, 1.42-3.11),8 and thrombocytopenia (OR, 1.79; 95% CI 0.97-3.29).8,32,33 When cutoffs for thrombocytopenia were assessed separately, results showed that there probably is a greater magnitude of association between risk of bleeding and platelet count <50 × 109/L compared with a platelet count ≥50 × 109/L (OR, 3.37; 95% CI, 1.84-6.18),26 whereas a smaller magnitude of association between risk of bleeding and platelet count <150 × 109/L compared with platelet count ≥150 × 109/L (OR, 1.30; 95% CI, 0.92-1.82).30,32 We also found moderate-certainty evidence that there is probably an association between risk of bleeding and blood dyscrasia, defined as the presence of any bleeding disorder on admission (OR, 1.70; 95% CI, 1.60-1.81),32 hepatic disease (OR, 1.53; 95% CI, 1.09-2.15),8,32 and renal failure (OR, 1.43; 95% CI, 1.06-1.93).8,32 One study assessed renal failure by severity and the results showed that there probably is a greater magnitude of association between risk of bleeding and severe renal failure (glomerular filtration rate [GFR] <30 mL/min/m2) vs no severe renal failure (OR, 2.14; 95% CI, 1.22-3.75)8 and a smaller magnitude of association between risk of bleeding and moderate renal failure (GFR, 30-59 mL/min/m2) compared with no moderate renal failure (OR, 1.37; 95% CI, 0.84-2.23).8 We also identified moderate-certainty evidence that there is probably an association between risk of bleeding and CVC use (OR, 1.37; 95% CI, 0.83-2.26)8,32 and antithrombotic medication use (OR, 1.28; 95% CI, 1.01-1.64).8,32
We found moderate-certainty evidence that there is probably little to no association between risk of bleeding and hormone use, defined as estrogen intake (OR, 0.95; 95% CI, 0.82-1.10).32
Low-quality evidence showed that there may be an association between risk of bleeding and autoimmune disease (OR, 1.30; 95% CI, 0.77-2.19).8,32 However, we identified low-certainty evidence that there may be little to no association between risk of bleeding and malignancy (OR, 1.08; 95% CI, 0.42-2.77).8,32
Laboratory and physical examination factors
There is moderate-certainty evidence of a probable association between risk of bleeding and anemia as the reason for admission (OR, 5.15; 95% CI, 2.45-10.81),8,33 morbid obesity (BMI ≥40 kg/m2; OR, 3.08; 95% CI, 1.35-7.02),33 and low hemoglobin (<13 g/dL in men and <11.5 g/dL in women; OR, 2.33; 95% CI, 1.04-5.22).33
Discussion
Summary of findings
We evaluated prognostic factors for VTE and bleeding in hospitalized medical patients. We identified 23 prognostic factors for VTE and 15 for bleeding, some supported by moderate certainty of the evidence. Age, critical illness, CVC use, and autoimmune disease were prognostic of both outcomes. Obesity (BMI >30 kg/m2) and morbid obesity (BMI >40 kg/m2) were associated with VTE and bleeding, respectively. However, only age, critical illness, and autoimmune disease had the same quality of evidence for the 2 outcomes. This study is unique in many aspects including its comprehensiveness, the novelty of its findings, and its transparent approach.
Strengths
Methodologically, our study benefits from the rigorous methods, the breadth of our search, our duplicate and independent screening, the data abstraction process used, and our assessment of the certainty of evidence on the basis of a structured framework. Also, we conducted sensitivity analyses to compare different duration cutoffs for immobility and to address differences in type of outcome. Other strengths include the involvement of a number of content and methodological experts.
Limitations and challenges
A potential limitation in terms of the search strategy was the focus on prevention, which we applied to restrict the rather large number of citations that we identified in our searches and because we believed that we would not miss relevant studies. To confirm this, we checked a large random sample (n = 3000) of citations obtained from a search not including a restriction to the topic of prevention. We did not identify any study that would have fulfilled the inclusion criteria and, therefore, our original search was unlikely to have missed eligible studies. Also, time bias may be a potential limitation, as we identified some of the prognostic factors from older studies. This may overestimate VTE events when considering overall trends in the reduction of VTE events in hospitalized medical patients over time.
Potential limitations of the included studies relate to the inconsistency and variability across eligibility criteria in the original studies and variability in study design, study type, sample size, and definitions of the prognostic factors. Other challenges include inconsistency in methods of measurement used across studies and contamination of the population with nonmedical hospitalized patients.
Implications for practice
Our study identified candidate prognostic factors for VTE and bleeding that have been considered in the analysis of some developed and widely used RAMs in daily practice, such as the Caprini, IMPROVE (International Medical Prevention Registry on Venous Thromboembolism) VTE, IMPROVE bleed, and Padua models.7,40-42 However, some factors that we identified as having a probable association with VTE, based on our meta-analysis results, were not included or considered in the development of most of the RAMs, such as elevated CRP >10 mg/L (OR, 10.10), lower BI scores (BI ≤ 9; OR, 8.30), history of malignancy (OR, 3.20), and elevated heart rate (>100 beats/min; OR, 2.48). In addition, we found that an elevated fibrinogen level was inversely associated with DVT risk in patients with early stroke.35 This observation was opposite to the finding that elevated CRP, another acute-phase prognostic factor, showed an association with DVT risk. The authors speculated that this finding may be a result of fibrinogen depletion due to active clot formation.35 We believe that such reverse causation, given the study design, may be plausible. However, given the small sample size, the finding warrants further investigations in primary studies. In terms of bleeding, the candidate prognostic factor antithrombotic use showed mixed results in the different studies and was included only in 1 final model.32 However, we identified a probable association with the outcome when the individual results were subjected to meta-analysis,8,25,33 perhaps because of the limitation of the databases used that may not include all potential prognostic factors. Another reason that their findings may have been limited is the methods used in the development of RAMs for the multivariate analysis, such as automated procedures (eg, backward or forward) used for prognostic factor selection or selection of factors based on statistical significance at the univariate analysis stage. Therefore, the findings in our study ensure the consideration of all identified potential prognostic factors in the literature during the development of a RAM, a better assessment of the databases being used, and the comprehensiveness of the factors included in the databases. Studies in this systematic review included patients who received thromboprophylaxis, which may have altered the risk estimates. However, we controlled for the use of thromboprophylaxis in several ways. We selected studies only if they included a small fraction of patients receiving thromboprophylaxis (less than 10%) or if it was controlled for in the statistical analysis. Beyond that, given the general assumption that the relative risk related to a prognostic factor remains largely unaffected by administration of thromboprophylaxis (although the baseline risk of course might change), we used relative estimates of the risk. This assumption is supported by the observation that, in several studies, the relative estimates of risk were not influenced by adjustment for prophylaxis use in the statistical analyses. We believe that these measures should address the influence of thromboprophylaxis on the prognostic factor we addressed herein.
Implications for future research
Research may be needed to reevaluate existing RAMs, as the developers of the models may not have been able to use the variables we identified, given the limitations in the existing databases. However, a full development or improvement of a RAM that supports clinical practice requires further investigation of all the prognostic factors we identified in our study.
Conclusion
In this systematic review, we identified all reported relevant prognostic factors for VTE and bleeding in hospitalized medical patients. Some of these factors are not part of current risk prediction for VTE and bleeding in hospitalized medical patients. Our findings will help inform experts in developing population-based guidelines and accurate, user-friendly RAMs to better guide individual patient prophylactic management.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a subcontract (200-2016-92458) from the US Centers for Disease Control and Prevention (CDC), through Karna LLC.
The CDC, through Karna LLC, had no role in the detailed design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation of the manuscript. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the CDC.
Authorship
Contribution: A.J.D. and H.J.S. were responsible for the conception and design of the study; A.J.D., S.G.K., R.C., I.E.-I., F.G., M.R., A.A., R.Z.M., and H.J.S. performed data acquisition; A.J.D., M.R., L.M., and H.J.S. performed data analyses; A.J.D., S.G.K., I.E.-I., M.C., M.K.G., L.M., F.A.S., A.C.S., M.B.S., S.W., N.A.Z., F.G., M.R., A.A., R.Z.M., A.I., E.A.A., and H.J.S. interpreted the results; A.J.D. and H.J.S. drafted the manuscript; and all authors critically revised the manuscript and approved the final version.
Conflict-of-interest disclosure: A.J.D., I.E.-I., A.I., E.A.A., and H.J.S. are members of the GRADE working group. A.C.S. has received remuneration for consulting from Bayer, Janssen, and Portola and research support grants from Boehringer Ingelheim, Janssen, and the Center for Medicare and Medicaid Services. A.C.S. also reported an intellectual conflict as the lead in the group that derived and validated the IMPROVE tool for VTE risk assessment in hospitalized medical patients. M.B.S. has received remuneration for consulting for Bayer, Janssen, Pfizer, and Portola and research support grants from Boehringer-Ingelheim, Janssen, Portola, and Roche. M.C. is a former board member (2013-2017) of the American Heart Association and chaired the committee that produced the American Society of Hematology (ASH) 2018 Guidelines for Management of Venous Thromboembolism: Prophylaxis for Hospitalized and Nonhospitalized Medical Patients (ASH 2018 Guidelines for Management of VTE). F.A.S. and N.A.Z. reported participating as panel members for the ASH 2018 Guidelines for Management of VTE. N.A.Z. also reported receiving honoraria in 2017 from ASH for the Highlights of ASH 2017 presentation (Dallas, New York, and Latin America). H.J.S. was cochair of the ASH 2018 Guidelines for Management of VTE committee and procured grant funding from the CDC for this study. The remaining authors declare no competing financial interests.
Correspondence: Holger J. Schünemann, Department of Health Research Methods, Evidence, and Impact, McMaster University, 1280 Main St West, Hamilton, ON L8N 3Z5, Canada; e-mail: schuneh@mcmaster.ca.

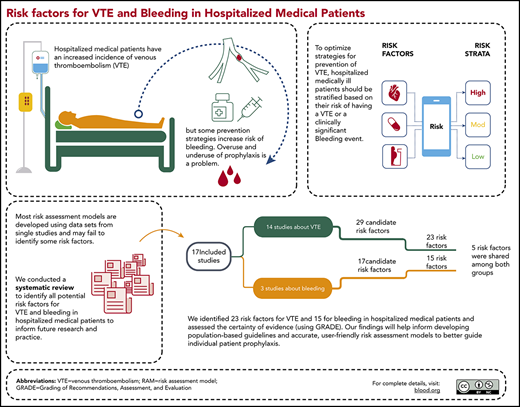

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal