In this issue of Blood, Markey et al demonstrate that alteration of intestinal microbiota and their short-chain fatty acid (SCFA) metabolites are associated with chronic graft-versus-host disease (cGVHD) after allogeneic stem cell transplantation (allo-SCT).1-10
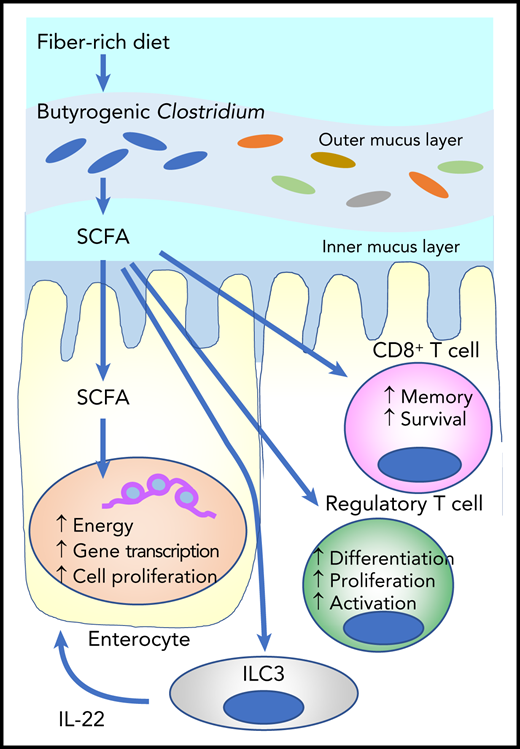
SCFAs such as butyrate are derived from fermentation of dietary fibers by SCFA-producing microbiota such as Clostridium. Butyrate provides the majority of the energy for enterocytes and promotes gene transcription and cell proliferation. It stimulates innate lymphoid cells (ILC3s) to produce interleukin-2 (IL-22), which enhances intestinal stem cell proliferation and differentiation. Butyrate also stimulates regulatory T-cell generation and activation, as well as effector T-cell functions.
SCFAs such as butyrate are derived from fermentation of dietary fibers by SCFA-producing microbiota such as Clostridium. Butyrate provides the majority of the energy for enterocytes and promotes gene transcription and cell proliferation. It stimulates innate lymphoid cells (ILC3s) to produce interleukin-2 (IL-22), which enhances intestinal stem cell proliferation and differentiation. Butyrate also stimulates regulatory T-cell generation and activation, as well as effector T-cell functions.
The gastrointestinal (GI) tract is the major reservoir of microbiota that play an important role in human health and disease. It is the major interface between microorganisms and the adoptive immune system. Emerging data suggest that alterations in the GI microbiota and metabolome are associated with the incidence and severity of acute GVHD (aGVHD) as well as transplant outcomes.2-4 However, most of the studies have focused on a short time after SCT (within 1 month), and data are not available for later time points when diet gradually returns to normal and cGVHD starts to develop.
Markey and colleagues analyzed stool and blood samples collected from inpatients and outpatients over time after allo-SCT. Loss of microbial diversity with predominant expansion of specific bacteria persisted for up to 1 year after SCT. However, the time course of the microbial alterations was similar in patients with cGVHD and controls. Then the authors focused on day 100 samples. Metagenomic sequencing of day 100 stool samples showed that microbial metabolic pathways related to SCFA metabolism were enriched in patients with cGVHD. Consistent with this, higher blood levels of butyrate and propionate SCFAs were associated with lower incidence of cGVHD.
SCFAs play an important role in the maintenance of health and the development of disease. They are the main metabolites produced by bacterial fermentation of dietary fiber in the GI tract (see figure). SCFAs such as butyrate, propionate, and acetate play an important role in intestinal homeostasis as the major energy source for enterocytes. They also stimulate group 3 innate lymphoid cells (ILC3s) to produce interleukin-22 (IL-22), a growth factor for intestinal stem cells.5 Butyrate mitigates aGVHD by protecting the energy homeostasis of enterocytes in experimental SCTs.6 A large-scale, international cooperative study of the fecal microbiota demonstrated that Enterococcus dominance with concomitant loss of butyrogenic Clostridium and fecal butyrate early after SCT was associated with increased aGVHD and reduced overall survival.3,4
Markey et al now show that increased relative abundance of butyrogenic Lachnoclostridium and Clostridium are associated with a reduced incidence of cGVHD. However, the role of butyrogenic bacteria is still controversial. A recent study demonstrated that increased relative abundance of butyrogenic bacteria after the onset of aGVHD was associated with subsequent steroid-refractory aGVHD or cGVHD, although the study was limited by small sample size.7 SCFAs can be both anti-inflammatory and pro-inflammatory by stimulating regulatory T-cell development and enhancing CD8+ T-cell effector and memory functions (see figure).8,9
Immune reconstitution after allo-SCT is characterized by a recapitulation of lymphoid ontogeny, and dysregulated immune reconstitution is associated with the development of cGVHD and the severity of cGVHD. Because development of the adoptive immune system in newborns requires healthy microbiota,10 it is possible that immune recapitulation after SCT also needs healthy microbiota. The results of the study by Markey et al suggest that dietary habit at home after SCT may be related to long-term transplant outcomes.
However, this study has several limitations. The number of samples is small, and most of the stool samples are from inpatients. It is not easy to regularly collect samples from outpatients, and therefore data can be biased. Because more than half the patients had overlap syndrome or late-onset aGVHD, results may reflect the acute GI-GVHD type, not classical cGVHD. This study highlights the current gap in cGVHD diagnosis. Except for esophageal involvement, GI manifestations in cGVHD are regarded as aGVHD.11 Furthermore, patients have often received antimicrobial and other pharmacologic agents that damage microbiota; data are not perfectly consistent between institutions in the Markey et al study.
The Markey et al study is just the beginning of understanding the mechanistic impact of the alteration of microbiota and their metabolites on cGVHD. Larger multi-institutional studies of the microbiome for an extended monitoring period, ideally until tolerance induction, are important for better understanding of the association between the microbiome and metabolome, and cGVHD or tolerance induction. Unfortunately, studies using stool samples are currently challenged by COVID-19 infection.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal