In this issue of Blood, Bender et al report the successful development of a model of light chain (LC) deposition disease using knockin κ LC gene in a transgenic mouse model.1 This model reproduces all the features of the human disease, but it does not rely on myeloma cells, which have been a double-edged sword in other models.
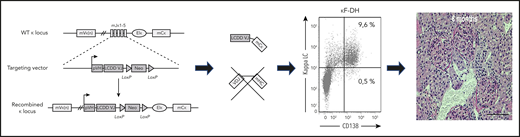
By replacing the Jκ segments of the mouse Ig gene with a human VJ exon/neomycin cassette, all endogenous rearrangement is blocked, which leads to the production of chimeric human VJ/mouse κ constant LC. Breeding with DH-LMP2A mice ensures the production of only free LCs by all B and plasma cells. The high serum concentrations of a monoclonal κ-free LC produced in the development of LC deposition disease is reminiscent of the human disease at 6 months.
By replacing the Jκ segments of the mouse Ig gene with a human VJ exon/neomycin cassette, all endogenous rearrangement is blocked, which leads to the production of chimeric human VJ/mouse κ constant LC. Breeding with DH-LMP2A mice ensures the production of only free LCs by all B and plasma cells. The high serum concentrations of a monoclonal κ-free LC produced in the development of LC deposition disease is reminiscent of the human disease at 6 months.
In 2012, the International Kidney and Monoclonal Gammopathy Research Group (IKMG) introduced the concept of monoclonal gammopathy of renal significance (MGRS).2 The concept of MGRS was based on monoclonal gammopathy of undetermined significance (MGUS), a premalignant condition, but there was 1 key difference. By definition, patients with MGUS cannot have any end organ damage, whereas patients with MGRS have a kidney disease associated with the monoclonal gammopathy.3 The goal of the MGRS designation by the IKMG was to separate the kidney disease from the cancer. Until then, treatment was predicated by the development of a malignancy.3 Conversely, there was an increasing recognition that patients were presenting with kidney injury from the monoclonal immunoglobulin (Ig) that did not meet the criteria for multiple myeloma. In some cases, such as chronic lymphocytic leukemia, kidney involvement was not even defined in the diagnostic criteria.4 Treatment was not recommended until standard criteria for the corresponding cancer was met, which were based on clonal proliferation and expansion.3,4 The introduction of MGRS showed that kidney disease can develop independently of the cancer, and the use of cytotoxic therapy to salvage or preserve kidney function was recommended, even when a cancer did not exist.2
The treatment of MGRS was built on 2 precedents.2 First, a majority of the patients with Ig LC (AL) amyloidosis have <10% bone marrow plasma cells with no myeloma-defining events, and few ever progress to multiple myeloma.5 Despite that, cytotoxic therapies, including autologous stem cell transplantation, are standard therapy for patients with AL amyloidosis.6 Second, studies have reproduced the kidney lesions in animals by injecting the human monoclonal Ig without any of the clonal cells, suggesting that the monoclonal protein, not the cells, is the most important pathogenic agent.7 This evidence suggests that elimination of monoclonal protein should be the goal and that the use of cytotoxic therapy is justified.
One of the most difficult aspects of studying MGRS-associated diseases is their rarity. AL amyloidosis, which has a prevalence of 10 to 12 per million per year is actually the most common MGRS-related disease.8 It is difficult to conduct a randomized trial in MGRS-related diseases, even for multicenter consortiums. Nearly all the treatment data come from retrospective studies. A good animal model is therefore invaluable for studying these diseases. However, creating a good animal model is not without challenge, and few animal models are actually successful in reproducing the human disease.9 One of the challenges in animal models is maintaining the level of monoclonal protein required for development of these renal lesions. The high sustained levels needed are often challenging to maintain, even with repeated injections. Second, in models in which the monoclonal protein is produced by myeloma cells, the animal may die of the malignancy before kidney disease develops. Finally, some animals have natural resistance to developing certain diseases. A prime example is AL amyloidosis, which is extremely difficult to reproduce in mice.9 Thus, only a limited number of animal models have been developed for studying these diseases.
So it is exciting that the group from Limoges, France, has successfully developed an animal model of MGRS-related disease, which is an advancement of their previous work. Their first model was a heavy chain (HC) deposition disease model, which was followed by an LC proximal tubulopathy (acquired Fanconi syndrome) model.9 Bender et al report the successful creation of a transgenic mouse model for LC deposition disease, the most common form of monoclonal Ig deposition disease. By using a site-directed insertion of a human pathologic LC gene into a mouse Ig κ locus, they were able to direct 95% of the mouse plasma cells to produce the human pathologic LC, which resulted in monoclonal free LC levels of 1000 mg/L in the serum and development of Bence Jones proteinuria similar to that in human patients (see figure). In addition, the LC deposition disease that developed in the mice mimics all of the human renal pathology, including nodular glomerulosclerosis, which could not be reproduced in the HC deposition disease model.9 Furthermore, treatment with cyclophosphamide and bortezomib resulted in reduction of the plasma cells and serum-free LC concentration, ultimately decreasing the LC deposits in the kidney, albuminuria, kidney injury, and death from kidney failure.
This transgenic mouse model represents a major breakthrough and will no doubt contribute to a better understanding of MGRS-related diseases. This model was able to reproduce the human pathology in mice, and it showed prevention of renal damage with treatment using cyclophosphamide and bortezomib, which are often used to treat patients with LC deposition disease. Most importantly, it provided unambiguous evidence that cancer, multiple myeloma in this case, is not required for the development of MGRS-related diseases. When the concept of MGRS was introduced, the goal was to gain access to cytotoxic therapies to eradicate the clone that produces the monoclonal protein to preserve kidney function.2 However, since the clones responsible for MGRS are usually indolent, it begs the question whether eradication of these small indolent clones is necessary if the monoclonal proteins can be removed or inhibited from interacting with the kidney.10 The ability to test this question was made possible with the model developed by Bender et al. Models like these are essential for developing new alternative treatments that do not rely on cytotoxic drugs. Ironically, that would take us back to the doctrine that cytotoxic therapy is reserved for cancer. Scientifically, that is actually a good thing, since ideas and concepts are never meant to be stagnant. They need to constantly be challenged so they can be improved.
Conflict-of-interest disclosure: The author declares no competing financial interests.