Key Points
Specific phospholipid-binding proteins and phospholipase eliminate muscle myosin-supported prothrombinase activity.
Previously unreported tissue factor-like activity, also present, contributes to myosin procoagulant function.
Abstract
Recent reports indicate that suspended skeletal and cardiac myosin, such as might be released during injury, can act as procoagulants by providing membrane-like support for factors Xa and Va in the prothrombinase complex. Further, skeletal myosin provides membrane-like support for activated protein C. This raises the question of whether purified muscle myosins retain procoagulant phospholipid through purification. We found that lactadherin, a phosphatidyl-l-serine–binding protein, blocked >99% of prothrombinase activity supported by rabbit skeletal and by bovine cardiac myosin. Similarly, annexin A5 and phospholipase A2 blocked >95% of myosin-supported activity, confirming that contaminating phospholipid is required to support myosin-related prothrombinase activity. We asked whether contaminating phospholipid in myosin preparations may also contain tissue factor (TF). Skeletal myosin supported factor VIIa cleavage of factor X equivalent to contamination by ∼1:100 000 TF/myosin, whereas cardiac myosin had TF-like activity >10-fold higher. TF pathway inhibitor inhibited the TF-like activity similar to control TF. These results indicate that purified skeletal muscle and cardiac myosins support the prothrombinase complex indirectly through contaminating phospholipid and also support factor X activation through TF-like activity. Our findings suggest a previously unstudied affinity of skeletal and cardiac myosin for phospholipid membranes.
Introduction
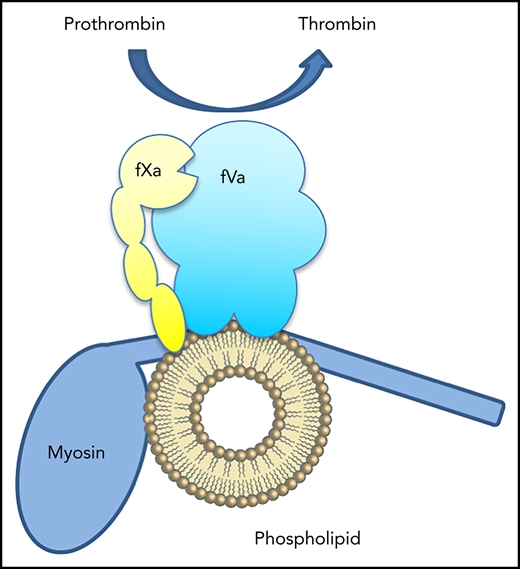
Recent reports indicate that both skeletal muscle (SkM) and cardiac muscle (CM) myosins support activity of the prothrombinase complex in the absence of phospholipid.1,2 The authors propose that myosin, particularly the neck region of the heavy chain, supports assembly of the prothrombinase complex in a manner analogous to phospholipid membranes.3 Another report from the same group shows that SkM myosin also enhances inactivation of factor Va by activated protein C (aPC).4 Thus, myosin is reported to serve in both a procoagulant and anticoagulant complex in a manner that is equivalent to phosphatidylserine-containing phospholipid membranes.
We have found that some membrane-binding proteins, notably factor V mutants, retain phospholipid from cell membranes through chromatography purification, leading to apparently membrane-independent activity in the prothrombinase assay.5 Earlier reports indicated that vitamin K-dependent coagulation proteins also retain bound membrane fragments through purification steps, leading to erroneous conclusions of apparent phospholipid-independent activity.6,7 Muscle myosin is prepared using a combination of precipitation/centrifugation steps and ion-exchange chromatography.8,9 Because this purification lacks a step to remove myosin-bound phospholipid, we hypothesized that contaminating phospholipid was the source of the reported procoagulant and anticoagulant activities.
Study design
Rabbit SkM and bovine CM myosins were purchased from Cytoskeleton, Inc. (Denver, CO). Human factors Va, VIIa, and Gla-domainless fXa (GD-fXa) were from Haematologic Technologies (Essex Junction, VT). Human factors X, Xa, II, and IIa were from Enzyme Research Laboratories (South Bend, IN). Chromogenix substrates S-2238 and S-2765 were from Diapharma (West Chester, OH). Phospholipids were purchased from Avanti Polar Lipids (Alabaster, AL), and vesicles prepared as previously described.10,11 Annexin A5 and honey bee phospholipase A2 (PLA2) were from Sigma-Aldrich (St. Louis, MO). Lactadherin was a generous gift from Jan Rasmussen. Tissue factor pathway inhibitor (TFPI) was a generous gift from Alan Mast.
Results and discussion
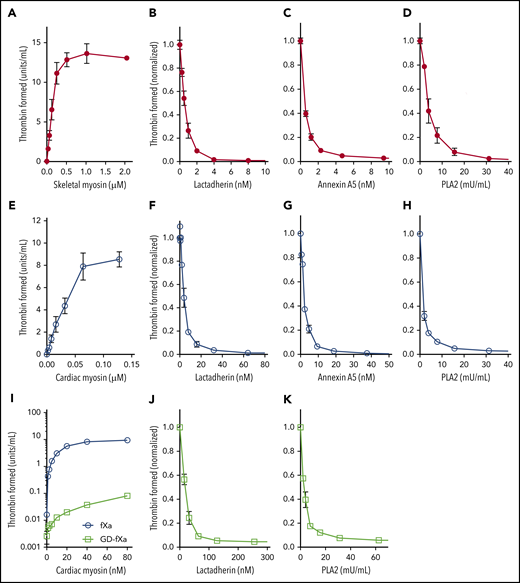
We first confirmed that SkM and CM myosin, prepared by the method and provided by the vendor previously used, support function of the prothrombinase complex (Figure 1A,E). We then asked whether lactadherin, a membrane-binding protein with stereospecific affinity for phosphatidyl-l-serine, would inhibit this activity (Figure 1B,F). Lactadherin inhibited >99% of myosin-dependent prothrombinase activity, with 50% inhibition at 0.33 nM for SkM and 4 nM for CM myosin. Because blocking phosphatidylserine-containing sites is the only mechanism through which lactadherin inhibits the prothrombinase complex,12 this result strongly suggests that contaminating phospholipid, containing phosphatidylserine, supports the prothrombinase complex. Annexin A5 is another phospholipid-binding protein that inhibits prothrombinase activity when membranes contain >4% phosphatidylserine and membrane convexity is limited12,13 ; it also inhibited >99% of myosin-dependent activity (Figure 1C,G). To further probe the role of contaminating phospholipid, we preincubated myosin with various concentrations of PLA2. We found that 30 mU/mL (5.5 nM) PLA2 decreased prothrombinase activity >95% (Figure 1D,H). The low concentrations of lactadherin and annexin A5 required to inhibit the prothrombinase complex imply a low content of contaminating phospholipid.1 Higher concentrations were needed to inhibit CM myosin activity, suggesting a greater degree of lipid contamination.
SkM and CM myosin-mediated prothrombinase activity results from contaminating phospholipids. Varying concentrations of rabbit SkM (A) or bovine CM (E) myosin were incubated with 5 nM fVa, 0.2 nM fXa, and 1 µM prothrombin in the presence of 5 mM CaCl2, 150 mM NaCl, and 50 mM Tris, pH 7.85. Reactions were stopped after 5 min with EDTA, fIIa substrate S-2238 was added, and color development was measured in kinetic mode on a VersaMax microplate reader (Molecular Devices). Substrate development was converted to U/mL IIa using a standard curve. Myosin supported generation of thrombin in the absence of added phospholipid. SkM, 64 nM, (B-D) or 16 nM CM (F-H) were preincubated with lactadherin (B,F), annexin A5 (C,G) or phospholipase A2 (D,H) for 5 min before the addition of fVa, fXa, and prothrombin. Lactadherin and annexin A5 inhibited myosin-mediated thrombin activation at similar concentrations (half-maximal inhibition: 0.35 ± 0.07 and 0.33 ± 0.02 nM, respectively, for SkM; 3.0 ± 0.8 and 1.4 ± 0.3 nM, respectively, for CM). Phospholipase concentrations >32 mU/mL inhibited SkM- and CM-mediated thrombin activation >99% and >95%, respectively. Support of CM for Gla-domain–deleted fXa (GD-fXa, 0.2 nM) or fXa for 10 min was evaluated (I). GD-fXa (open squares) supported <1% of the activity supported by full fXa (open circles). To test inhibition in the presence of GD-fXa, CM (160 nM) was incubated with either lactadherin (J) or PLA2 (K) for 5 min before the addition of 5 nM fVa, 1 nM GD-fXa, and 1 µM prothrombin. The reaction was run for 10 min before quenching with EDTA. Lactadherin and PLA2 inhibited ∼95% of myosin-mediated activity. Data shows mean ± standard deviation (SD) for 4 (SkM) and 3 (CM) experiments, each performed in duplicate.
SkM and CM myosin-mediated prothrombinase activity results from contaminating phospholipids. Varying concentrations of rabbit SkM (A) or bovine CM (E) myosin were incubated with 5 nM fVa, 0.2 nM fXa, and 1 µM prothrombin in the presence of 5 mM CaCl2, 150 mM NaCl, and 50 mM Tris, pH 7.85. Reactions were stopped after 5 min with EDTA, fIIa substrate S-2238 was added, and color development was measured in kinetic mode on a VersaMax microplate reader (Molecular Devices). Substrate development was converted to U/mL IIa using a standard curve. Myosin supported generation of thrombin in the absence of added phospholipid. SkM, 64 nM, (B-D) or 16 nM CM (F-H) were preincubated with lactadherin (B,F), annexin A5 (C,G) or phospholipase A2 (D,H) for 5 min before the addition of fVa, fXa, and prothrombin. Lactadherin and annexin A5 inhibited myosin-mediated thrombin activation at similar concentrations (half-maximal inhibition: 0.35 ± 0.07 and 0.33 ± 0.02 nM, respectively, for SkM; 3.0 ± 0.8 and 1.4 ± 0.3 nM, respectively, for CM). Phospholipase concentrations >32 mU/mL inhibited SkM- and CM-mediated thrombin activation >99% and >95%, respectively. Support of CM for Gla-domain–deleted fXa (GD-fXa, 0.2 nM) or fXa for 10 min was evaluated (I). GD-fXa (open squares) supported <1% of the activity supported by full fXa (open circles). To test inhibition in the presence of GD-fXa, CM (160 nM) was incubated with either lactadherin (J) or PLA2 (K) for 5 min before the addition of 5 nM fVa, 1 nM GD-fXa, and 1 µM prothrombin. The reaction was run for 10 min before quenching with EDTA. Lactadherin and PLA2 inhibited ∼95% of myosin-mediated activity. Data shows mean ± standard deviation (SD) for 4 (SkM) and 3 (CM) experiments, each performed in duplicate.
Des-Gla fXa (GD-Xa) lacks the membrane-binding Gla domain. It has reduced activity that, reportedly, is enhanced by fVa, but independent of phospholipid.14 Both SkM and CM myosins supported limited activity of GD-Xa in prior reports, and this was interpreted as proof of phospholipid independence for the myosin activites.1,2 We verified that CM myosin supports activity of GD-fXa at levels somewhat below 1% of activity of intact fXa (Figure 1I). However, lactadherin (Figure 1J) and PLA2 (Figure 1K) inhibited myosin-mediated GD-fXa by 95%, indicating that this activity is also largely a result of contaminating phospholipid. Our results are not adequate to explain the apparent conflict with prior results that indicate phospholipid independence of fVa-enhanced activity of GD-Xa. However, we note that the report of phospholipid independence for GD-Xa in the prothrombinase complex used a single composition of phospholipid that was not representative of a cell membrane.14 Thus, the general assumptions about phospholipid independence may have been overly broad. Further, because the activity of GD-Xa is greatly reduced, trace contamination by intact fXa could lead to apparent increased activity and phospholipid dependence. We did not test for contaminating intact fXa, and such testing was not included in prior reports.
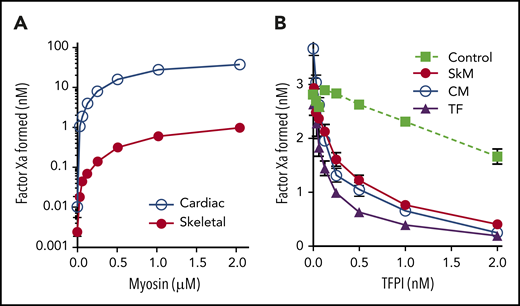
Although phospholipid contamination explains the prothrombinase and aPC-anticoagulant activity of myosin in purified assays, it may not completely explain the enhancement of thrombin generation demonstrated in plasma.1,2 We therefore speculated that the contaminating membranes might retain the dominant extravascular procoagulant, tissue factor (TF).15-17 TF serves as a cofactor for fVIIa in cleavage of fX in the extrinsic factor Xase complex. We therefore mixed SkM and CM myosins with fVIIa and fX to test for extrinsic Xase activity. Our results show that the myosin preparations facilitated fXa production (Figure 2A) over the background amount of fX cleaved by vesicle-bound fVIIa (0.048 ± 0.005 nM fXa). Using relipidated human TF as a control, we estimated that 1 μM SkM myosin has activity equivalent to 7 ± 1 pM units of human TF, whereas 1 µM CM myosin has the equivalent of 320 ± 10 pM TF activity. This corresponds well to the quantity of TF previously added to plasma thrombin generation assays as a comparator to the role of myosin procoagulant activity.1 This is also consistent with prior reports indicating that CM preparations contain ∼20-fold more TF than SkM.18 Unfortunately, we were unable to locate inhibitory antibodies against rabbit or bovine TF for confirmatory testing. However, we confirmed that preincubation of either CM or SkM myosin with 2 nM TFPI inhibited this activity >76% (versus 86% inhibition with human TF) confirming TF-like function (Figure 2B). Thus, both SkM and CM myosin preparations have previously overlooked TF-like activity that is probably a result of TF embedded in contaminating phospholipid membranes.
SkM and CM myosins exhibit TF-like activity. Varying concentrations of myosin were mixed with 1 nM fVIIa and 150 nM fX for 5 min before quenching with EDTA. FXa generation was then measured using substrate S-2765. (A) SkM (1 µM, red circles) supported generation of fXa equivalent to 7.3 pM TF based on a control curve of relipidated human TF, wheras 1 µM CM myosin (blue open circles) supported generation of fXa equivalent to 320 pM TF. (B) TFPI was preincubated with either relipidated, recombinant human TF (7.3 pM, purple triangles), SkM (1 µM, red circles), or CM (23.7 nM, open circles) for 5 minutes. fVIIa and fX were then added, and fXa generation was assessed after 30 min. Control experiments lacking TF (green squares) were run by incubating 3 nM fXa and 1 nM fVIIa with TFPI for 30 min and then testing activity. TFPI over 2 nM inhibits fXa activity >50%. However, TFPI inhibits myosin-mediated fXa activation by an additional 76% (SkM) and 84% (CM) over its effect on fXa (inhibition of TF-mediated fXa activation is 87%). Data shown are mean ± SD for 3 (SkM) and 2 (CM) experiments, each performed in duplicate.
SkM and CM myosins exhibit TF-like activity. Varying concentrations of myosin were mixed with 1 nM fVIIa and 150 nM fX for 5 min before quenching with EDTA. FXa generation was then measured using substrate S-2765. (A) SkM (1 µM, red circles) supported generation of fXa equivalent to 7.3 pM TF based on a control curve of relipidated human TF, wheras 1 µM CM myosin (blue open circles) supported generation of fXa equivalent to 320 pM TF. (B) TFPI was preincubated with either relipidated, recombinant human TF (7.3 pM, purple triangles), SkM (1 µM, red circles), or CM (23.7 nM, open circles) for 5 minutes. fVIIa and fX were then added, and fXa generation was assessed after 30 min. Control experiments lacking TF (green squares) were run by incubating 3 nM fXa and 1 nM fVIIa with TFPI for 30 min and then testing activity. TFPI over 2 nM inhibits fXa activity >50%. However, TFPI inhibits myosin-mediated fXa activation by an additional 76% (SkM) and 84% (CM) over its effect on fXa (inhibition of TF-mediated fXa activation is 87%). Data shown are mean ± SD for 3 (SkM) and 2 (CM) experiments, each performed in duplicate.
Previous studies report that fXa binds to myosin and that binding may be mediated by a peptide within the neck region.3 Our data do not give a reason to question the binding of fXa to myosin. However, our previous findings raise a cautionary note about the use of constituent peptides to localize specific protein–protein interactions for membrane-binding proteins. We and others have found that peptides capable of forming amphipathic helices can bind to phospholipid membranes.19 Curiously, the amphipathic helices can also mimic the function of a phospholipid bilayer, enhancing fIXa activity.20 Because the putative fXa binding peptide of SkM is part of an α-helical region21 with 54% hydrophobic amino acids,3 binding of fXa may be related to the amphipathic and helical character of the isolated peptide rather than the specific amino acids.
Our results indicate that prothrombinase-supporting activity of SkM and CM myosin depends upon contaminating phospholipid. We also note that the myosin preparations contain TF-like activity that has not previously been reported. Binding to phospholipid membranes has previously been described for nonmuscle myosin II,22-24 and we hypothesize that muscle myosin may share this property as a mechanism to explain the residual procoagulant phospholipid in myosin preparations.1,4 Additional studies will likely indicate the details of the interaction between phospholipid membranes and muscle myosin.
Primary data will be shared upon e-mail request.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christopher V. Carman and Donna J. Brezinski for feedback on the manuscript.
This work was supported by a Department of Veterans Affairs Merit Award to G.E.G.
Authorship
Contribution: G.E.G. proposed the experiments and assisted with writing and editing the manuscript; and V.A.N. conducted experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gary E. Gilbert, Department of Veterans Affairs, Harvard Medical School, 150 South Huntington Ave, Boston, MA 02132; e-mail: gary_gilbert@hms.harvard.edu.