Key Points
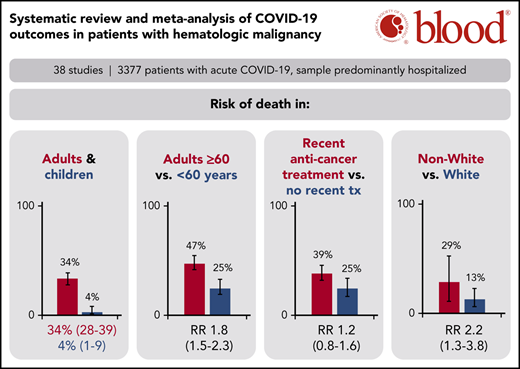
Adult patients with hematologic malignancy and COVID-19 found a 34% risk of death, whereas pediatric patients had a 4% risk of death.
Patients on systemic anticancer therapy had a similar risk of death to patients on no treatment (RR, 1.17; 95% CI, 0.83-1.64).
Abstract
Outcomes for patients with hematologic malignancy infected with COVID-19 have not been aggregated. The objective of this study was to perform a systematic review and meta-analysis to estimate the risk of death and other important outcomes for these patients. We searched PubMed and EMBASE up to 20 August 2020 to identify reports of patients with hematologic malignancy and COVID-19. The primary outcome was a pooled mortality estimate, considering all patients and only hospitalized patients. Secondary outcomes included risk of intensive care unit admission and ventilation in hospitalized patients. Subgroup analyses included mortality stratified by age, treatment status, and malignancy subtype. Pooled prevalence, risk ratios (RRs), and 95% confidence intervals (CIs) were calculated using a random-effects model. Thirty-four adult and 5 pediatric studies (3377 patients) from Asia, Europe, and North America were included (14 of 34 adult studies included only hospitalized patients). Risk of death among adult patients was 34% (95% CI, 28-39; N = 3240) in this sample of predominantly hospitalized patients. Patients aged ≥60 years had a significantly higher risk of death than patients <60 years (RR, 1.82; 95% CI, 1.45-2.27; N = 1169). The risk of death in pediatric patients was 4% (95% CI, 1-9; N = 102). RR of death comparing patients with recent systemic anticancer therapy to no treatment was 1.17 (95% CI, 0.83-1.64; N = 736). Adult patients with hematologic malignancy and COVID-19, especially hospitalized patients, have a high risk of dying. Patients ≥60 years have significantly higher mortality; pediatric patients appear to be relatively spared. Recent cancer treatment does not appear to significantly increase the risk of death.
Introduction
A substantial number of guidance documents and review articles have been published regarding the management of patients with cancer and the novel severe acute respiratory syndrome coronavirus 2 (COVID-19).1-10 However, there are no systematic reviews or meta-analyses specific to patients with hematologic malignancies. These patients are recognized to be highly immunocompromised due to their underlying disease as well as the treatments they receive, causing significant concern about a risk of heightened morbidity and mortality from COVID-19 in this population. On the other hand, some authors have suggested that some patients with hematologic malignancies might be “protected” from severe COVID-19 morbidity due to an attenuated inflammatory response.11-13 Cohort and registry studies have emerged to answer these and other questions, including the COVID-19 and Cancer Consortium (CCC19), the UK Coronavirus Cancer Monitoring Project (UKCCMP), and the American Society of Hematology (ASH) Research Collaborative.
Given the rapidly evolving literature and overall limited data in patients with hematologic malignancy, aggregating data to obtain more precise estimates of the risks related to COVID-19 is essential to inform clinical decision-making. The objective of this study was to perform a systematic review and meta-analysis to quantify the outcomes (deaths, hospitalizations, and complications) of patients with hematologic malignancy and COVID-19.
Methods
This study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Eligibility criteria
All studies published since 1 January 2019 on outcomes of patients with cancer and COVID-19 were considered for inclusion. Only studies providing data on patients with hematologic malignancy (bone marrow failure syndromes such as myelodysplastic syndromes [MDS], acute leukemias, lymphomas, plasma cell dyscrasias, and/or myeloproliferative neoplasms [MPNs]) were included. Both adult (age ≥18 years) and pediatric (age <18 years) studies were included. Case reports, case series, or cohort studies with <10 patients were excluded. Only English and Chinese language reports were included. Full inclusion criteria are available in supplemental Table 1 (available on the Blood Web site).
Information sources and search strategy
PubMed and EMBASE databases were searched up to the week of 17 August 2020. The full search strategy is available in supplemental Table 2.
Two authors (A.V. and I.Y.G.) independently conducted the search strategy, and results were compared to ensure concordance. Differences in opinion were discussed and resolved, with a third author (L.K.H.) available for resolution of disagreements. Titles and abstracts of articles were reviewed and any that were clearly irrelevant were excluded. Full texts of remaining articles were reviewed to find studies that met the inclusion criteria. Additionally, systematic reviews related to cancer and COVID-19 were screened to identify additional references.
Data collection
A data-extraction form was used to extract relevant information from the articles. Information extracted was specific to patients with hematologic malignancy, and included geographic location of study, total number of patients, median age, distribution by sex, and total study duration, as well as whether follow-up was complete, death rate, death rate in inpatients, intensive care unit (ICU) admission rate, mechanical ventilation rate, noninvasive ventilation rate (continuous positive airway pressure, bilevel positive airway pressure, high-flow oxygen by nasal cannula), and death rate stratified by treatment, age, and hematologic malignancy subtype.
For treatment subgroups, “systemic anticancer therapy” (SACT) was defined as patients on active anticancer therapy (ie, cytotoxic chemotherapy, immunotherapy, targeted agents; single-agent hydroxyurea for MPNs and steroids were excluded from this definition) within 28 days to 6 months of COVID-19 diagnosis (depending on varying definitions used in each study). A subgroup of SACT was defined as “cytotoxic SACT” and included patients on cytotoxic therapy only (eg, multiagent systemic chemotherapy or antimyeloma therapy; excluding single-agent immunotherapy, single-agent targeted therapy, single-agent hydroxyurea for MPNs, or steroids). “Not on treatment” was defined as patients on observation or those for whom it had been >28 days to 6 months since their last active treatment. “Best supportive care” (BSC) was defined as patients on supportive care only, such as hydroxyurea alone for acute leukemia, erythropoietin-stimulating agents, or patients who were on BSC as indicated in studies.
Hematologic malignancy subtypes were divided as follows: acquired bone marrow failure syndromes (eg, MDS, aplastic anemia); acute leukemias (myeloid and lymphoid); lymphomas (non-Hodgkin and Hodgkin); plasma cell dyscrasias (multiple myeloma, amyloidosis, smoldering myeloma, monoclonal gammopathy of undetermined significance); and MPNs (chronic myeloid leukemia, polycythemia vera, essential thrombocytosis, myelofibrosis). In select cases for which key data were not included, authors of studies were e-mailed for clarification of the published data.
Risk of bias in individual studies
As the majority of studies included were descriptive cohort studies with no comparator arm, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Studies Reporting Prevalence Data was used (see supplemental Table 3 for checklist).14 Sample-size adequacy was assessed using previously described methods,15 using an estimated risk of death of 0.35 and precision of 0.05 indicating a confidence interval (CI) width of 10%. Studies scoring at least 6 of 9 were considered low risk, as previously reported.16 Assessment was conducted by 2 authors (A.V. and I.Y.G); a third author (L.K.H.) was available to resolve differences of opinion.
Outcomes
The primary outcome of the meta-analysis was the pooled risk of death among patients with hematologic malignancies and COVID-19, subdivided into adult and pediatric patients. Risks of death in all patients as well as within hospitalized patients are reported.
Secondary outcomes included the proportion of hospitalized patients requiring ICU admission and ventilation support (mechanical and noninvasive). Prespecified subgroup analyses were conducted for pooled risk of death stratified by age, race (non-White vs White), treatment status, hematologic malignancy subtype, and geographic location (Asia vs Europe vs North America). Prespecified sensitivity analyses were conducted on the primary outcomes, limiting to studies with low risk of bias, to studies with complete follow-up of all patients, to studies diagnosing COVID-19 based solely on real-time polymerase chain reaction (RT-PCR), and to studies that included a combination of outpatients and hospitalized patients. Due to data limitations, secondary outcomes, subgroup analyses, and sensitivity analyses were not completed for the studies reporting on pediatric patients.
Synthesis of results
The principal summary measures used were pooled prevalence and risk ratios (RR) with 95% CIs. Heterogeneity between estimates was assessed using the I2 statistic, and interpreted per the Cochrane Handbook recommendations: I2 of 0% to 40%, heterogeneity likely not substantial; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; 75% to 100%, considerable heterogeneity.17 For the primary outcome, secondary outcomes, and subgroup analyses, estimates were transformed using the Freeman-Tukey double arcsine method,18 and the final pooled results were back-transformed with 95% CI for ease of interpretation. For secondary outcomes involving RR, pooled dichotomous-effect measures were expressed as RR with 95% CI. Meta-analysis was performed using a random-effects model (DerSimonian and Laird) using the MetaXL (www.epigear.com) add-in for Microsoft Excel, as well as Review Manager 5.4 (Cochrane Collaboration 2020).
Publication bias was assessed using the Doi plot and the Luis Furuya-Kanamori asymmetry index (LFK index).19 The closer the value of the LFK index to 0, the more symmetrical the Doi plot (ie, low risk of publication bias). LFK index values outside of the interval between −1 and +1 are consistent with asymmetry (ie, publication bias). Sensitivity testing was performed to assess the main source of asymmetry.
Results
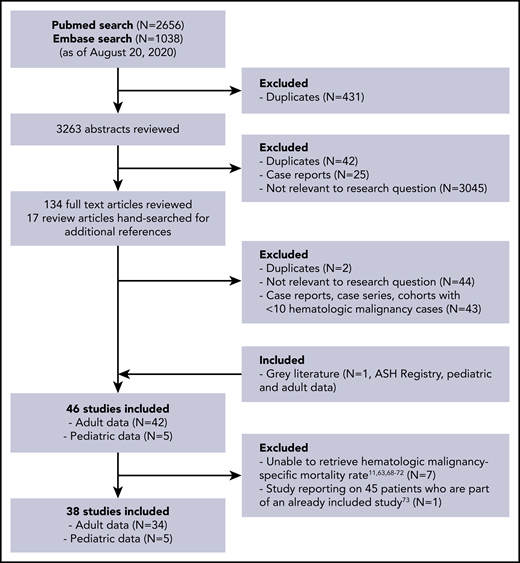
Figure 1 shows the flow diagram for study selection. A total of 34 adult studies (32 peer-reviewed, 1 preprint, 1 open online registry) and 5 pediatric studies (4 peer-reviewed, 1 open online registry) comprising 3377 patients from Asia, Europe, and North America were included.20-57 Total duration of studies ranged from 3 weeks to 15 weeks. COVID-19 was diagnosed based solely on RT-PCR in the majority of studies (27 of 38 sources); others did not specify (4 of 38) or used clinical suspicion, imaging, or external reporting in some patients (7 of 38). The majority of data are regarding patients followed at hospitals or cancer centers; 1 study used a countrywide Ministry of Health database.52 Table 1 lists the summary characteristics of the included studies. Table 2 lists the results of the risk-of-bias assessment for individual studies, and supplemental Table 4 includes details of the scoring. When assessing for publication bias, major asymmetry was noted (LFK index, 2.18) (supplemental Figure 1); this was largely driven by the only study that used population-based data.52 The LFK index, when excluding this study due to the differences in methodology, was 0.94, indicating no asymmetry and a low risk of publication bias.
Characteristics of included studies
| First author/year . | Location . | Type of malignancy included . | Duration of study . | Total no. of pts with HM . | Total no. of pts with HM hospitalized . | Median age of pts with HM, y . | Female pts with HM, N (%) . | Died, N (%) . | No. of pts who had not yet recovered/ unknown status at end of study . |
|---|---|---|---|---|---|---|---|---|---|
| Adult studies | |||||||||
| Aries20 /2020* | Europe | All | 2 mo | 35 | 24 | 69 | 12 (34) | 14 (40) | 0 |
| Biernat21 /2020 | Europe | All | 1 mo | 10 | 10 | 58 | 8 (80) | 7 (70) | 0 |
| Booth22 /2020* | Europe | All | 2 mo | 66 | 66 | 73 | 25 (38) | 34 (52) | 4 |
| Cook23 /2020* | Europe | Myeloma | 15 wk | 75 | 72 | 73 | 30 (40) | 41 (55) | NR |
| Dufour24 /2020* | Europe | Myeloma | NR | 20 | 18 | 68 | 8 (40) | 7 (35) | NR |
| Engelhardt25 /2020 | Europe | Myeloma | 3 mo | 21 | 17 | 59 | 4 (19) | 0 (0) | NR |
| Fattizzo26 /2020* | Europe | All | 6 wk | 16 | 13 | 77 | 6 (38) | 5 (31) | NR |
| Ferrara27 /2020 | Europe | AML | 1 mo | 10 | 10 | 60 | 5 (50) | 5 (50) | 1 |
| Fox28 /2020* | Europe | All | 1 mo | 54 | 51 | 63 | 18 (33) | 19 (35) | 1 |
| He29 /2020 | Asia | All | 3 wk | 13 | 13 | 35 | 6 (46) | 8 (62) | 0 |
| Hultcrantz30 /2020 | North America | Myeloma | 7 wk | 100 | 74 | 68 | 42 (42) | 18 (18) | NR |
| Infante31 /2020 | Europe | All | 1 mo | 41 | 29 | 76 | 19 (47) | 15 (37) | NR |
| Kuderer32 /2020 | Multicenter | All | 1 mo | 204† | 104 | NR | NR | 24 (14) | NR |
| Lattenist33 /2020* | Europe | All | 2 mo | 12 | 12 | 74 | 3 (25) | 6 (50) | NR |
| Lee34 /2020 | Europe | All | 6 wk | 169 | NR | NR | NR | 60 (36) | 0 |
| Malard35 /2020 | Europe | All | 1 mo | 25 | 25 | 72 | 8 (32) | 10 (40) | NR |
| Martín-Moro36 /2020* | Europe | All | 5 wk | 34 | 34 | 73 | 15 (44) | 11 (32) | 5 |
| Mato37 /2020 | Multicenter | CLL | 2.5 mo | 198 | 178 | 71 | 73 (37) | 66 (33) | 49 |
| Mehta38 /2020* | North America | All | 3 wk | 54 | 54 | NR | NR | 20 (37) | NR |
| Mei39 /2020 | Asia | All | Unclear | 13 | 13 | NR | NR | 6 (46) | NR |
| Passamonti40 /2020 | Europe | All | 12 wk | 536 | 451 | 68 | 196 (37) | 198 (37) | 11 |
| Patell41 /2020* | North America | All | 2 mo | 19 | 19 | NR | NR | 13 (68) | 4 |
| Razanamahery42 /2020* | Europe | All | 8 wk | 20 | 20 | 69 | 7 (35) | 6 (30) | NR |
| Rugge43 /2020 | Europe | All | 5 wk | 81 | 48 | NR | NR | 13 (16) | NR |
| Russell44 /2020 | Europe | All | 10 wk | 28 | NR | NR | NR | 7 (25) | NR |
| Sanchez-Pina45 /2020 | Europe | All | 1 mo | 39 | 34 | 65 | 16 (41) | 14 (40) | 19 |
| Scarfò46 /2020* | Europe | CLL | 10 wk | 190 | 169 | 72 | 64 (34) | 56 (29) | 38 |
| Shah47 /2020 | Europe | All | 8 wk | 80 | 80 | 73 | 28 (35) | 28 (35) | 5 |
| Tian48 /2020 | Asia | All | 9 wk | 12 | 12 | NR | 5 (42) | 5 (42) | NR |
| Varma49 /2020 | North America | All | NR | 34 | 25 | 57 | 12 (35) | 7 (21) | NR |
| Wang50 /2020 | North America | Myeloma | 2 mo | 58 | 36 | 67 | 28 (48) | 14 (24) | 0 |
| Yang51 /2020 | Asia | All | 2 mo | 22 | 22 | 55 | 7 (32) | 9 (41) | 0 |
| Yigenoglu52 /2020 | Asia | All | 15 wk | 740 | 452 | 56 | 343 (46) | 102 (14) | NR |
| ASH registry53 /2020* | Multicenter | All | Ongoing (3 mo at time of data extraction) | 264‡ | 176 | NR | 106 (40) | 74 (30) | 16 |
| Pediatric studies | |||||||||
| Bisogno54 /2020 | Europe | All | 2 mo | 20 | NR | NR | NR | 0 (0) | 0 |
| de Rojas55 /2020 | Europe | All | NR | 11 | NR | 11 | 1 (9) | 0 (0) | NR |
| Faura56 /2020 | Europe | All | 3 mo | 41 | NR | NR | NR | 2 (5) | NR |
| Ferrari57 /2020 | Europe | All | 8 wk | 12 | NR | NR | NR | 0 (0) | NR |
| ASH registry53 /2020* | Multicenter | All | Ongoing (3 mo at time of data extraction) | 18 | NR | NR | NR | 2 (11) | 2 |
| First author/year . | Location . | Type of malignancy included . | Duration of study . | Total no. of pts with HM . | Total no. of pts with HM hospitalized . | Median age of pts with HM, y . | Female pts with HM, N (%) . | Died, N (%) . | No. of pts who had not yet recovered/ unknown status at end of study . |
|---|---|---|---|---|---|---|---|---|---|
| Adult studies | |||||||||
| Aries20 /2020* | Europe | All | 2 mo | 35 | 24 | 69 | 12 (34) | 14 (40) | 0 |
| Biernat21 /2020 | Europe | All | 1 mo | 10 | 10 | 58 | 8 (80) | 7 (70) | 0 |
| Booth22 /2020* | Europe | All | 2 mo | 66 | 66 | 73 | 25 (38) | 34 (52) | 4 |
| Cook23 /2020* | Europe | Myeloma | 15 wk | 75 | 72 | 73 | 30 (40) | 41 (55) | NR |
| Dufour24 /2020* | Europe | Myeloma | NR | 20 | 18 | 68 | 8 (40) | 7 (35) | NR |
| Engelhardt25 /2020 | Europe | Myeloma | 3 mo | 21 | 17 | 59 | 4 (19) | 0 (0) | NR |
| Fattizzo26 /2020* | Europe | All | 6 wk | 16 | 13 | 77 | 6 (38) | 5 (31) | NR |
| Ferrara27 /2020 | Europe | AML | 1 mo | 10 | 10 | 60 | 5 (50) | 5 (50) | 1 |
| Fox28 /2020* | Europe | All | 1 mo | 54 | 51 | 63 | 18 (33) | 19 (35) | 1 |
| He29 /2020 | Asia | All | 3 wk | 13 | 13 | 35 | 6 (46) | 8 (62) | 0 |
| Hultcrantz30 /2020 | North America | Myeloma | 7 wk | 100 | 74 | 68 | 42 (42) | 18 (18) | NR |
| Infante31 /2020 | Europe | All | 1 mo | 41 | 29 | 76 | 19 (47) | 15 (37) | NR |
| Kuderer32 /2020 | Multicenter | All | 1 mo | 204† | 104 | NR | NR | 24 (14) | NR |
| Lattenist33 /2020* | Europe | All | 2 mo | 12 | 12 | 74 | 3 (25) | 6 (50) | NR |
| Lee34 /2020 | Europe | All | 6 wk | 169 | NR | NR | NR | 60 (36) | 0 |
| Malard35 /2020 | Europe | All | 1 mo | 25 | 25 | 72 | 8 (32) | 10 (40) | NR |
| Martín-Moro36 /2020* | Europe | All | 5 wk | 34 | 34 | 73 | 15 (44) | 11 (32) | 5 |
| Mato37 /2020 | Multicenter | CLL | 2.5 mo | 198 | 178 | 71 | 73 (37) | 66 (33) | 49 |
| Mehta38 /2020* | North America | All | 3 wk | 54 | 54 | NR | NR | 20 (37) | NR |
| Mei39 /2020 | Asia | All | Unclear | 13 | 13 | NR | NR | 6 (46) | NR |
| Passamonti40 /2020 | Europe | All | 12 wk | 536 | 451 | 68 | 196 (37) | 198 (37) | 11 |
| Patell41 /2020* | North America | All | 2 mo | 19 | 19 | NR | NR | 13 (68) | 4 |
| Razanamahery42 /2020* | Europe | All | 8 wk | 20 | 20 | 69 | 7 (35) | 6 (30) | NR |
| Rugge43 /2020 | Europe | All | 5 wk | 81 | 48 | NR | NR | 13 (16) | NR |
| Russell44 /2020 | Europe | All | 10 wk | 28 | NR | NR | NR | 7 (25) | NR |
| Sanchez-Pina45 /2020 | Europe | All | 1 mo | 39 | 34 | 65 | 16 (41) | 14 (40) | 19 |
| Scarfò46 /2020* | Europe | CLL | 10 wk | 190 | 169 | 72 | 64 (34) | 56 (29) | 38 |
| Shah47 /2020 | Europe | All | 8 wk | 80 | 80 | 73 | 28 (35) | 28 (35) | 5 |
| Tian48 /2020 | Asia | All | 9 wk | 12 | 12 | NR | 5 (42) | 5 (42) | NR |
| Varma49 /2020 | North America | All | NR | 34 | 25 | 57 | 12 (35) | 7 (21) | NR |
| Wang50 /2020 | North America | Myeloma | 2 mo | 58 | 36 | 67 | 28 (48) | 14 (24) | 0 |
| Yang51 /2020 | Asia | All | 2 mo | 22 | 22 | 55 | 7 (32) | 9 (41) | 0 |
| Yigenoglu52 /2020 | Asia | All | 15 wk | 740 | 452 | 56 | 343 (46) | 102 (14) | NR |
| ASH registry53 /2020* | Multicenter | All | Ongoing (3 mo at time of data extraction) | 264‡ | 176 | NR | 106 (40) | 74 (30) | 16 |
| Pediatric studies | |||||||||
| Bisogno54 /2020 | Europe | All | 2 mo | 20 | NR | NR | NR | 0 (0) | 0 |
| de Rojas55 /2020 | Europe | All | NR | 11 | NR | 11 | 1 (9) | 0 (0) | NR |
| Faura56 /2020 | Europe | All | 3 mo | 41 | NR | NR | NR | 2 (5) | NR |
| Ferrari57 /2020 | Europe | All | 8 wk | 12 | NR | NR | NR | 0 (0) | NR |
| ASH registry53 /2020* | Multicenter | All | Ongoing (3 mo at time of data extraction) | 18 | NR | NR | NR | 2 (11) | 2 |
AML, acute myeloid leukemia; ASH, American Society of Hematology; CLL, chronic lymphocytic leukemia; HM, hematologic malignancy; NR, not reported; pts, patients; UK, United Kingdom.
Authors who provided extra information via e-mail communication.
Outcome data only available on 167 patients.
Outcome data only available on 248 patients.
Risk-of-bias assessments
| Study: first author/year . | Total score . | Low risk of bias . |
|---|---|---|
| Aries20 /2020 | 7 | ✓ |
| Biernat21 /2020 | 7 | ✓ |
| Booth22 /2020 | 6 | ✓ |
| Cook23 /2020 | 7 | ✓ |
| Dufour24 /2020 | 5 | |
| Engelhardt25 /2020 | 6 | ✓ |
| Fattizzo26 /2020 | 6 | ✓ |
| Ferrara27 /2020 | 6 | ✓ |
| Fox28 /2020 | 6 | ✓ |
| He29 /2020 | 6 | ✓ |
| Hultcrantz30 /2020 | 7 | ✓ |
| Infante31 /2020 | 6 | ✓ |
| Kuderer32 /2020 | 6 | ✓ |
| Lattenist33 /2020 | 5 | |
| Lee34 /2020 | 8 | ✓ |
| Malard35 /2020 | 7 | ✓ |
| Martín-Moro36 /2020 | 5 | |
| Mato37 /2020 | 6 | ✓ |
| Mehta38 /2020 | 7 | ✓ |
| Mei39 /2020 | 1 | |
| Passamonti40 /2020 | 7 | ✓ |
| Patell41 /2020 | 8 | ✓ |
| Razanamahery42 /2020 | 5 | |
| Rugge43 /2020 | 6 | ✓ |
| Russell44 /2020 | 6 | ✓ |
| Sanchez-Pina45 /2020 | 7 | ✓ |
| Scarfò46 /2020 | 6 | ✓ |
| Shah47 /2020 | 8 | ✓ |
| Tian48 /2020 | 6 | ✓ |
| Varma49 /2020 | 6 | ✓ |
| Wang50 /2020 | 7 | ✓ |
| Yang51 /2020 | 8 | ✓ |
| Yigenoglu52 /2020 | 7 | ✓ |
| ASH registry53 /2020 | 2 |
| Study: first author/year . | Total score . | Low risk of bias . |
|---|---|---|
| Aries20 /2020 | 7 | ✓ |
| Biernat21 /2020 | 7 | ✓ |
| Booth22 /2020 | 6 | ✓ |
| Cook23 /2020 | 7 | ✓ |
| Dufour24 /2020 | 5 | |
| Engelhardt25 /2020 | 6 | ✓ |
| Fattizzo26 /2020 | 6 | ✓ |
| Ferrara27 /2020 | 6 | ✓ |
| Fox28 /2020 | 6 | ✓ |
| He29 /2020 | 6 | ✓ |
| Hultcrantz30 /2020 | 7 | ✓ |
| Infante31 /2020 | 6 | ✓ |
| Kuderer32 /2020 | 6 | ✓ |
| Lattenist33 /2020 | 5 | |
| Lee34 /2020 | 8 | ✓ |
| Malard35 /2020 | 7 | ✓ |
| Martín-Moro36 /2020 | 5 | |
| Mato37 /2020 | 6 | ✓ |
| Mehta38 /2020 | 7 | ✓ |
| Mei39 /2020 | 1 | |
| Passamonti40 /2020 | 7 | ✓ |
| Patell41 /2020 | 8 | ✓ |
| Razanamahery42 /2020 | 5 | |
| Rugge43 /2020 | 6 | ✓ |
| Russell44 /2020 | 6 | ✓ |
| Sanchez-Pina45 /2020 | 7 | ✓ |
| Scarfò46 /2020 | 6 | ✓ |
| Shah47 /2020 | 8 | ✓ |
| Tian48 /2020 | 6 | ✓ |
| Varma49 /2020 | 6 | ✓ |
| Wang50 /2020 | 7 | ✓ |
| Yang51 /2020 | 8 | ✓ |
| Yigenoglu52 /2020 | 7 | ✓ |
| ASH registry53 /2020 | 2 |
COVID-19–associated mortality: adult studies
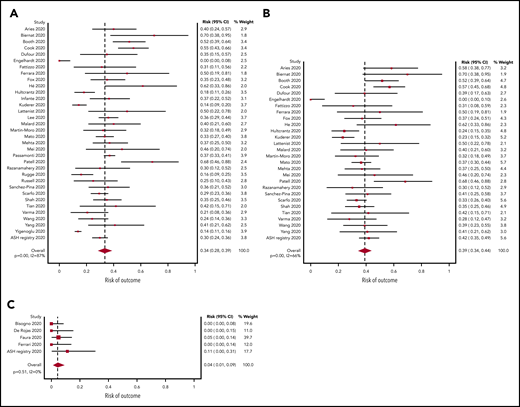
A total of 34 studies (3240 patients) had data available regarding mortality associated with a diagnosis of COVID-19 in adult patients with hematologic malignancy. The pooled risk of death was 34% (95% CI, 28-39) (Figure 2A). Substantial heterogeneity was detected (I2, 87%). When limiting the analysis to mortality among inpatients only, a total of 28 studies with 2361 hospitalized patients showed a pooled risk of 39% (95% CI, 34-44), with decreased heterogeneity (I2, 66%) (Figure 2B).
Pooled risk of mortality. (A) In all studies. (B) In hospitalized patients only. (C) Pooled risk of mortality in pediatric patients.
Pooled risk of mortality. (A) In all studies. (B) In hospitalized patients only. (C) Pooled risk of mortality in pediatric patients.
COVID-19–associated mortality: pediatric studies
The pooled risk of death was 4% (95% CI, 1-9) in pediatric studies (5 studies, 102 patients) (Figure 2C). No statistically significant heterogeneity was detected (I2, 0%).
ICU admission and ventilation
Twenty-four studies (2192 patients) provided data regarding need for ICU admission among hospitalized patients, 21 studies (1320 patients) provided data regarding need for mechanical ventilation in hospitalized patients, and 12 studies (373 patients) provided data regarding need for noninvasive ventilation in hospitalized patients. For ICU admission, the pooled risk was 21% (95% CI, 16-27; I2, 87%); for mechanical ventilation, the pooled risk was 17% (95% CI, 13-21; I2, 63%); for noninvasive ventilation, the pooled risk was 16% (95% CI, 9-26; I2, 79%) (supplemental Figure 2).
Subgroup and sensitivity analyses
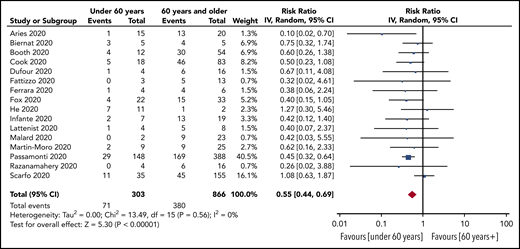
Prespecified age-stratified (<60 years and ≥60 years) subgroup analyses were conducted. Data with age were available from 16 studies (1169 patients). Patients under 60 years of age had a lower pooled risk of mortality (25%; 95% CI, 19-33, I2, 24%) compared with patients aged 60 years and older (47%; 95% CI, 41-54; I2, 58%). The pooled RR for death for patients under 60 years vs patients aged 60 and older was 0.55 (95% CI, 0.44-0.69; P < .00001), with no statistically significant heterogeneity (I2, 0%) (Figure 3).
RR of death in patients aged under 60 years vs aged 60 years and older.
Subgroup analysis based on race using data from 5 studies that reported race-based outcomes (162 patients) showed that non-White patients had a significantly higher risk of death compared with White patients (RR, 2.2 [95% CI, 1.3-3.8; P = .003; I2, 0%]) (supplemental Figure 3).
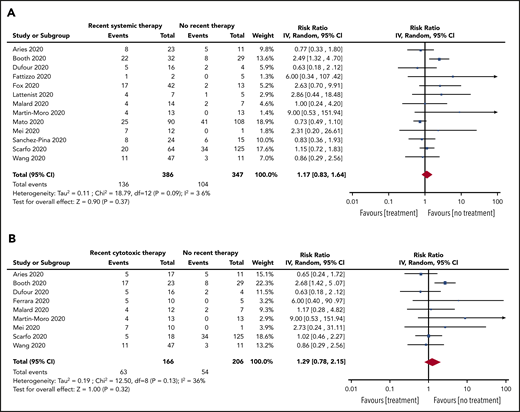
Subgroup analyses were also conducted for patients with recent SACT (19 studies, 736 patients), the subgroup of cytotoxic SACT (13 studies, 614 patients), patients not on treatment (14 studies, 356 patients), and patients on BSC (5 studies, 21 patients). The pooled risk of mortality was 39% (95% CI, 32-46; I2, 67%), 40% (95% CI, 32-47; I2, 68%), 25% (95% CI, 17-34; I2, 53%), and 85% (95% CI, 67-97; I2, 0%) for recent SACT, the subgroup cytotoxic SACT, no treatment, and BSC, respectively. The pooled RR for death for patients with recent SACT compared with no treatment was 1.17 (95% CI, 0.83-1.64; P = .37), with no statistically significant heterogeneity (I2, 36%; P = .09) (Figure 4A). When restricting the analysis to the subgroup of patients with recent cytotoxic SACT vs no treatment, the pooled RR of death was 1.29 (95% CI, 0.78-2.15; P = .32), with no statistically significant heterogeneity (I2, 36%; P = .15) (Figure 4B).
RR of death in patients. (A) On systemic anticancer therapy vs on no treatment. (B) On cytotoxic systemic anticancer therapy vs on no treatment.
RR of death in patients. (A) On systemic anticancer therapy vs on no treatment. (B) On cytotoxic systemic anticancer therapy vs on no treatment.
Pooled risk of death was also calculated by hematologic malignancy subtype. The following list outlines the risk of death for:
acquired bone marrow failure syndromes (14 studies, 231 patients), 53% (95% CI, 34-72; I2, 77%);
acute leukemias (18 studies, 289 patients), 41% (95% CI, 30-52; I2, 57%);
plasma cell dyscrasias (23 studies, 412 patients), 33% (95% CI, 25-41; I2, 58%);
lymphomas, including CLL (20 studies, 1324 patients), 32% (95% CI, 24-40; I2, 43%);
lymphomas, excluding CLL (14 studies, 485 patients), 32% (95% CI, 18-48; I2, 65%);
CLL specifically (15 studies, 517 patients), 31% (95% CI, 23-40; I2, 52%); and
MPNs (12 studies, 293 patients), 34% (95% CI, 19-51; I2, 73%) (supplemental Figure 4).
Sensitivity analysis including only studies with a lower risk of bias showed a similar estimate for risk of death among all patients (33% [95% CI, 27-39; I2, 89%], 28 studies with 2893 patients) compared with all studies. Sensitivity analysis including only studies with complete follow-up for all patients showed a similar risk of death compared with all studies (40% [95% CI, 30-51; I2, 58%], 6 studies with 307 patients). Sensitivity analysis including only studies that diagnosed COVID-19 using RT-PCR showed a similar estimate for risk of death among all patients compared with all studies (33% [95% CI, 26-39; I2, 90%]; 24 studies with 2674 patients). Sensitivity analysis including only studies that reported on a combination of outpatients and hospitalized patients showed a similar estimate for risk of death among all patients (31% [95% CI, 24-39; I2, 92%]; 18 studies with 2407 patients) compared with all studies.
Discussion
We report the first meta-analysis to date of the risk of death in patients with hematologic malignancies and COVID-19, incorporating data from 3377 patients from 3 continents. The estimates of mortality are most applicable for hospitalized patients as the majority of patients included in this analysis were hospitalized (77%). The pooled risk of mortality in all adult patients was 34% (95% CI, 28-39), whereas the pooled risk of mortality limited to hospitalized patients alone was 39% (95% CI, 34-44). Furthermore, patients aged 60 years and older had a significantly higher risk of death than patients under 60 years (47% vs 25%; RR, 1.82; 95% CI, 1.45-2.27; P < .00001), though the risk in both age groups was substantial. On the other hand, the pooled risk of death in pediatric patients was significantly lower than adult patients at 4% (95% CI, 0-8), confirming previous reports that increasing age is highly correlated with risk of death from COVID-19.58,59 Why age is such a powerful correlate of COVID mortality has not been determined. Theories include the possibility that children are less prone to a hyperinflammatory immune response compared with adults, as well as differences in their angiotensin-converting enzyme 2 distribution that may limit viral entry and subsequent inflammation, hypoxia, and tissue injury.60
The adult mortality rate reported in this meta-analysis appears substantially higher than in patients with solid tumors, or in the general population; however, the context of these data are important. The majority (77%) of the patients in our analysis were hospitalized and 14 of 34 adult studies included only hospitalized patients. In cohort studies exclusively of hospitalized patients with cancer, the mortality rate ranges from 19% to 42% in patients with solid tumor.38,42 The risk of death in hospitalized patients without cancer was 21% to 22% in large studies from New York and Germany,61,62 including 36% in patients aged ≥60 years.62 Thus, the risk of death in hospitalized patients with hematologic malignancy of 39% found in our analysis is comparable to hospitalized patients with solid tumor, but remains substantially higher than in the general population. The comparable risk of death to patients with solid tumor supports the notion that patients with hematologic malignancy should not be excluded from more intensive supportive care for COVID-19 solely on the basis of their hematologic diagnosis.
To ascertain the true risk of mortality among all patients with hematologic malignancy and COVID-19 (including all outpatients), it will be important for studies to collect data on an unselected population of patients. The largest study included in this meta-analysis, by Yigenoglu and colleagues from Turkey,52 likely has the best estimate for the true population mortality risk for patients with hematologic malignancy infected with COVID-19 (14%), as they used population-based data from a countrywide Ministry of Health database. This estimate remains higher than the risk of death for a control population in their study (7%),52 and the risk reported in a previous meta-analysis including noncancer inpatients and outpatients with COVID-19 (8%).5 The risk estimate of 14% reported by Yigenoglu is also comparable to the estimated risk of death of 13% in patients with all cancers.5
There is concern that recent SACT may result in inferior outcomes in patients with COVID-19. However, our analysis did not show evidence that recent SACT conferred a statistically significant excess risk of death compared with no treatment (RR, 1.22; 95% CI, 0.84-1.78; P = .29). This finding persisted even when limiting the analysis to a subgroup of patients on recent cytotoxic SACT (RR, 1.29; 95% CI, 0.78-2.15; P = .32). This is consistent with reports from other large studies of patients with cancer.32,34,63 This finding may be related to recent observations that patients with therapy-induced anergy of the immune system might have a milder form of COVID-19. In fact, some therapies tested in treating COVID-19 are hematologic/immunosuppressive drugs.64 Although it is sensible to withhold or delay SACT where disease kinetics permit, these data suggest that in patients who require urgent therapy for their hematologic malignancy, treatment can be delivered despite the risks of COVID-19. However, the analysis should be considered with caution given the heterogeneity of definitions of “recent treatment” among included studies. Clinicians should make decisions on a case-by-case basis with their patients, considering the community prevalence of COVID-19 in their region and the availability of health care resources.
Finally, we did find that race was an important contributor to mortality, with non-White patients having a significantly higher risk of mortality than White patients, consistent with previous reports.65,66 We do not know whether the differences in mortality reflect an inherent biologic risk of poor outcome, impact of comorbidities, impact of social determinants of health, vs implicit bias in the provision of health care.
Following the outbreak of COVID-19, many hospitals, particularly in Europe, opened clinical areas where high-level care interventions such as noninvasive ventilation could be delivered to mitigate shortages of ICU beds. The establishment of such high-dependency areas outside of a traditional ICU setting made the risk of ICU admission difficult to quantify and introduced substantial heterogeneity in our analysis. A previous meta-analysis showed a risk of ICU admission of 38% among all patients with cancer, utilizing a modified definition of ICU admission to include these high dependency clinical areas.5
This study has several important limitations. First, there is the possibility of duplicate patients within studies. We are aware of 2 studies with overlap of 3 patients,24,33 and 3 studies from centers20,22,28 that report data to the UKCCMP; thus duplicate patients may potentially have been reported by Lee et al.34 Although it is not known which centers contributed to the ASH registry, the registry was not accepting batch data until recently, making overlap between other large aggregate data efforts unlikely (L.K.H., written personal communication, 19 September 2020). Additionally, the majority of studies included were from different centers, different regions, or described differing diagnoses, thus we feel that duplicate reporting is unlikely to be a major factor in our meta-analysis.
A more important limitation of our work is the significant heterogeneity that was observed in many of the reported pooled estimates of mortality. In particular, the pooled overall mortality estimate had substantial heterogeneity (I2, 87%). This likely reflects the diverse nature of included patients including inpatients vs outpatients, wide age ranges, diverse hematologic diagnoses, and varied treatment practices across geographic areas. We sought to explore the observed heterogeneity through subgroup analyses. Our findings suggest that age is an important contributor to heterogeneity. When patients <60 years vs ≥60 years were analyzed separately, or pediatric patients were analyzed, heterogeneity substantially decreased. It is also likely that the primary hematologic diagnosis contributed to heterogeneity, as stratified analyses by diagnosis also decreased heterogeneity. Thus, our pooled estimates of overall mortality should be interpreted with caution pending the publication of additional primary data.
An additional limitation of this report is the possibility that mortality may be overestimated due to the included cohort studies being enriched with hospitalized patients and patients with frequent medical visits. Fourteen of the 34 adult reports in this meta-analysis included exclusively hospitalized patients. Moreover, even in those studies that included ambulatory patients, case ascertainment was usually dependent on the patients intersecting with the medical system: healthier, asymptomatic, or pauci-symptomatic patients may thus be underrepresented in the included sample. This bias may result in an overestimate of the risk of dying from COVID-19 among patients with hematologic malignancy. Additionally, many of the included studies report outcomes from the earliest phases of the pandemic; it is possible that mortality rates will improve due to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients. On the other hand, several studies included in this sample had insufficient follow-up to determine the final vital status of all patients in their sample (Table 1), introducing potential bias in the other direction.
A final limitation of our study relates to the fact that mortality reported in the included studies was assumed to be related to the diagnosis of COVID-19 given the short interval follow-up and highest risk of death from COVID-19 within weeks of the diagnosis; however, we acknowledge that certain hematologic malignancies (eg, acute leukemia) are also immediately life-threatening. However, a previous study found that the risk of mortality in inpatients with hematologic malignancy increases by 50% if they are infected with COVID-19.21
During the COVID-19 pandemic, gathering, analyzing, and reporting outcome data are more important than ever for specific at-risk patient populations. The rate at which data on clinical outcomes of COVID-19 in cancer patients is being collected and published is remarkable; within a 10-week period between our initial and final search strategy execution, over 1600 new studies were published. This pace of publication presents a challenge for clinicians, researchers, and guideline committees to assimilate the latest findings. Meta-analyses such as this are critical in order to analyze outcomes in larger cohorts of patients and to assess trends across specific at-risk groups.
We report a systematic review and meta-analysis of the literature regarding the risk of mortality in patients with hematologic malignancy and COVID-19, current to 20 August 2020. We report a high risk of death in this population (34%), partially owing to a large percentage of hospitalized patients in studies published to date. Nonetheless, our findings highlight the importance of preventing COVID-19 among patients with hematologic malignancy. Evidence-based prevention strategies such as infection-control measures, physical distancing, and appropriate shielding advice should be emphasized for hematology patients and the units in which they receive their care.67 Importantly, despite a concerning risk of death, a majority of patients with hematologic malignancy and COVID-19 recover, even following recent SACT. As a result, we recommend that hematology patients with COVID-19 should be considered for intensive supportive interventions where appropriate and if consistent with patient preference. Finally, our data suggest that among patients who require urgent treatment of a hematologic malignancy, treatment should not be routinely withheld due to a fear of excess mortality from COVID-19.
Take-home points for clinical practice regarding patients with hematologic malignancy and COVID
Mortality appears to be high, estimated at 34%; however, the estimate may be biased by a high number of hospitalized patients in published studies
Age is strongly associated with mortality: among those >60 years, mortality is estimated at 47% (95% CI, 41% to 54%); among those <18 years, mortality is estimated at 4% (95% CI, 1% to 9%)
Non-White patients appear to experience higher mortality than White patients
Recent systemic anticancer therapy may not impact mortality
Most patients with hematologic malignancy and COVID survive
All data are reported in the paper in figures or supplemental figures. Aggregate data tables are available from the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors sincerely thank all authors of all studies included in this meta-analysis, including those who provided extra data. The authors thank Benjamin Djulbegovic for assistance with select statistical analyses. The authors additionally acknowledge important contributors to work in this area including Javier Lopez-Jimenez, Jean Cyr Yombi, Amit Verma, and Juri Giannotta.
Authorship
Contribution: A.V. performed literature search, article selection, analysis, and manuscript writing; I.Y.G. performed article selection and manuscript review and revision; T.A.F., B.F., G.C., J.R., F.M.-M., J.C.R., J.Z., R.P., M.C.V., H.Y., R.L., L.S., T.C., S.B., I.M., and W.A.W. provided data clarifications and manuscript review and revision; and L.K.H. conceived of the study, assisted with analysis, and reviewed and revised the manuscript.
Conflict-of-interest disclosure: G.C. has received research funding from Takeda, Celgene, Janssen, and IQVIA, and has provided consultancy for Takeda, Celgene, Janssen, Sanofi, Amgen, Roche, and Karyopharm. B.F. has received consultation fees from Momenta Pharmaceuticals and Apellis SRL on autoimmune hemolytic anemia. J.Z. has received research funding from Incyte and Quercegen; has provided consultancy for Sanofi, CSL, and Parexel; and has received honoraria or served on advisory boards for Pfizer/Bristol Myers Squibb, Portola, and Dova. L.S. has received honoraria from AbbVie, AstraZeneca, Gilead, and Janssen. W.A.W. has received research funding from Pfizer and Genentech, is a consultant for Best Doctors/Teladoc, is an advisor for and holds equity in Koneksa Health and Elektra Labs, and has received honoraria from the ASH Research Collaborative. L.K.H. is co–principal investigator on a study partially funded by Gilead Sciences. The remaining authors declare no competing financial interests.
Correspondence: Lisa K. Hicks, St. Michael’s Hospital, Room 2-084 Donnelly Wing, 30 Bond St, Toronto, ON M5B 1W8, Canada; e-mail: lisak.hicks@unityhealth.to.