TO THE EDITOR:
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily manifests as a respiratory illness and has affected >5 million people worldwide, with >350 000 deaths.1,2 There are no current approved therapies for COVID-19. Administration of convalescent plasma (CP) may be effective therapy for COVID-19.3-6 Early indicators suggest that transfusion of CP is safe in COVID-19.7 We report the early clinical experience of 20 hospitalized patients treated with CP compared with 20 matched controls with severe or life-threatening COVID-19 infection.
Twenty patients with COVID-19 infection, diagnosed using quantitative reverse-transcriptase polymerase chain reaction assay for SARS-CoV-2 on nasopharyngeal swabs, were treated with CP in 5 hospitals in the Seattle area between April 13 and April 26 of 2020. Patients with severe or critical illness were treated with 1 unit of ABO-compatible CP under an expanded access protocol (IND 19832). Baseline demographic and clinical characteristics, including comorbidities, severity of illness, laboratory parameters, and clinical outcomes, were recorded up to 14 days after CP transfusion or the equivalent day of hospitalization for controls. Donor information was also collected. This study was approved by the Institutional Review Board of Providence St. Joseph Health.
The median age of patients treated with CP was 60 years (range, 29-95), with 20% of patients older than 80 years. The most common signs and symptoms of COVID-19 illness were cough or shortness of breath (90%), lymphopenia (67%), and an abnormal radiograph (80%). The most common comorbidities were hypertension (60%), diabetes (45%), and obesity (20%). One third of patients required mechanical ventilation (MV). Median time from hospitalization to CP was early at 2 days (interquartile range [IQR], 1-4.3). The majority of patients received additional therapies, including azithromycin (60%), hydroxychloroquine (55%), or multiple combinations. CP recipients had primarily A (45%) or B (45%) ABO type.
The 8 COVID-19–recovered donors who provided units ranged in age from 29 to 79 years. All had symptoms of respiratory illness, muscle aches, and/or headache, but none required hospitalization. All were more >28 days past their last symptoms of COVID illness. Donations were well tolerated and without significant complications. Anti–SARS-CoV-2 immunoglobulin G serology, as determined by the Abbott ARCHITECT, was positive in all but 1 donor, whereas EuroImmun was negative (n = 1), equivocal (n =3), moderate positive (n = 3), or high positive (n = 1).
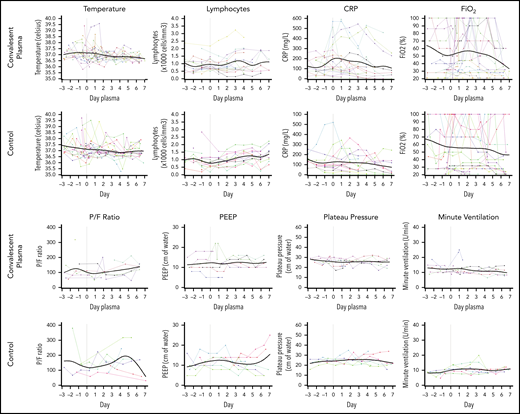
Figure 1 shows the clinical and laboratory parameters reported over 7 days after CP. Temperature improved in all patients after delivery of CP. The mean temperature decrease was 0.3°C in the CP group (standard deviation [SD], 0.5), whereas mean absolute lymphocyte count was 1.01 × 103/μL (SD, 0.47) and did not change by day 7 (1.09 × 103/μL; SD, 0.55). The mean baseline C-reactive protein was 170.5 mg/L (SD, 137.6), which decreased to 127.5 mg/L (SD, 144.0) after CP. A decrease in the fraction of inspired oxygen (FiO2) from 53% (SD, 29) to 47% (SD, 24) was seen by day 7 after CP. For patients who were on MV, the mean Pao2/FiO2 of inspired oxygen ratio at baseline was 81 (SD, 23), which improved to 96 (SD, 22). Two patients were liberated from the ventilator, whereas 2 required intubation after CP.
Serial change in laboratory and clinical parameters in CP and control patients. Solid lines represent a smoothing spline through the observed data points. CRP, C-reactive protein; PEEP, positive end-expiratory pressure; P/F, Pao2/FiO2.
Serial change in laboratory and clinical parameters in CP and control patients. Solid lines represent a smoothing spline through the observed data points. CRP, C-reactive protein; PEEP, positive end-expiratory pressure; P/F, Pao2/FiO2.
The outcomes of all patients are reported in Table 1. Median World Health Organization (WHO) ordinal scale score was 5 at CP infusion, which improved to 4.5 at day 7 and 3.5 at day 14. No adverse events with CP were reported. The incidence of venous thromboembolism (VTE) was (20%). At 7 days of follow-up, 25% of patients were discharged, whereas 10% had died. The 2 deaths occurred in patients who had been intubated for >2 weeks each and chose to transition to comfort measures. No patients died if they received CP prior to 7 days of hospitalization. No additional deaths occurred in the CP group by day 14.
Clinical outcomes
| Clinical status . | CP (n = 20) . | Matched controls (n = 20) . | ||||
|---|---|---|---|---|---|---|
| Baseline (d 0) . | Status at 7-d follow-up . | Status at 14-d follow-up . | Baseline* . | Status at 7-d follow-up . | Status at 14-d follow-up . | |
| WHO ordinal scale score | ||||||
| Median (IQR) | 5 (4.0-6.3) | 4.5 (4.0-6.0) | 3.5 (0-6) | 5 (4-7) | 4.5 (0.8-7.3) | 3 (0-8) |
| Mean (SD) | 5.2 (1.3) | 5.07 (1.4) | 3.1 (3.1) | 5.4 (1.4) | 4.2 (3.2) | 3.45 (3.6) |
| VTE | 4 (20) | 4 (20) | 4 (20) | 4 (20) | 4 (20) | 4 (20) |
| ICU | ||||||
| MV | 6 (30) | 6 (30) | 4 (20) | 6 (30) | 5 (25) | 1 (5) |
| Duration of MV (IQR), d | 14.5 (6-17.8) | 11.5 (7.5-9.8) | 16.5 (12.8-20) | 3 (2.3-3.8) | 9 (7-0) | 11 (8.5-13.5) |
| Extubated survivors | — | 1 (5) | 2 (10) | — | 0 (0) | 3 (15) |
| Adverse event after CP | 0 (0) | 0 (0) | 0 (0) | — | — | — |
| Hospital stay, d | ||||||
| LOS prior to CP, median (range) | 2 (1-21) | — | — | — | — | — |
| LOS prior to CP, median (IQR) | 2 (1-4.3) | — | — | — | — | — |
| Total LOS, median (IQR) | — | 9 (8-11.3) | 15 (10-16.5) | — | 8 (7-9.5) | 9 (7-13.5) |
| LOS of nonsurvivors, median (IQR) | — | 19.5 (15.8-23.3) | 19.5 (15.8-23.3) | — | 8 (7-9) | 8.5 (7.3-10.5) |
| LOS of survivors, median (IQR) | — | 9 (8-10) | 15 (10-16) | — | 8 (7-9.5) | 10 (7.3-15) |
| Discharged | — | 5 (25) | 9 (45) | — | 7 (35) | 9 (45) |
| Deaths | — | 2 (10) | 2 (10) | — | 5 (25) | 6 (30) |
| CP given prior to 7 d of hospitalization | — | 0 (0) | 0 (0) | — | 4 (20) | 5 (25) |
| CP given after 7 d of hospitalization | — | 2 (10) | 2 (10) | — | 1 (5) | 1 (5) |
| Clinical status . | CP (n = 20) . | Matched controls (n = 20) . | ||||
|---|---|---|---|---|---|---|
| Baseline (d 0) . | Status at 7-d follow-up . | Status at 14-d follow-up . | Baseline* . | Status at 7-d follow-up . | Status at 14-d follow-up . | |
| WHO ordinal scale score | ||||||
| Median (IQR) | 5 (4.0-6.3) | 4.5 (4.0-6.0) | 3.5 (0-6) | 5 (4-7) | 4.5 (0.8-7.3) | 3 (0-8) |
| Mean (SD) | 5.2 (1.3) | 5.07 (1.4) | 3.1 (3.1) | 5.4 (1.4) | 4.2 (3.2) | 3.45 (3.6) |
| VTE | 4 (20) | 4 (20) | 4 (20) | 4 (20) | 4 (20) | 4 (20) |
| ICU | ||||||
| MV | 6 (30) | 6 (30) | 4 (20) | 6 (30) | 5 (25) | 1 (5) |
| Duration of MV (IQR), d | 14.5 (6-17.8) | 11.5 (7.5-9.8) | 16.5 (12.8-20) | 3 (2.3-3.8) | 9 (7-0) | 11 (8.5-13.5) |
| Extubated survivors | — | 1 (5) | 2 (10) | — | 0 (0) | 3 (15) |
| Adverse event after CP | 0 (0) | 0 (0) | 0 (0) | — | — | — |
| Hospital stay, d | ||||||
| LOS prior to CP, median (range) | 2 (1-21) | — | — | — | — | — |
| LOS prior to CP, median (IQR) | 2 (1-4.3) | — | — | — | — | — |
| Total LOS, median (IQR) | — | 9 (8-11.3) | 15 (10-16.5) | — | 8 (7-9.5) | 9 (7-13.5) |
| LOS of nonsurvivors, median (IQR) | — | 19.5 (15.8-23.3) | 19.5 (15.8-23.3) | — | 8 (7-9) | 8.5 (7.3-10.5) |
| LOS of survivors, median (IQR) | — | 9 (8-10) | 15 (10-16) | — | 8 (7-9.5) | 10 (7.3-15) |
| Discharged | — | 5 (25) | 9 (45) | — | 7 (35) | 9 (45) |
| Deaths | — | 2 (10) | 2 (10) | — | 5 (25) | 6 (30) |
| CP given prior to 7 d of hospitalization | — | 0 (0) | 0 (0) | — | 4 (20) | 5 (25) |
| CP given after 7 d of hospitalization | — | 2 (10) | 2 (10) | — | 1 (5) | 1 (5) |
Unless otherwise noted, all data are n (%).
LOS, length of stay; —, not applicable.
Baseline day 0 for matched controls corresponds to equivalent hospital day as CP transfusion.
Control patients (n = 20) were well matched with regard to age, number of comorbidities, WHO score, sequential organ failure assessment score, and severity of illness. The median WHO score improved from 5 to 4.5 at day 7 and 3 at day 14. Half of the control patients received remdesivir (RDV). VTE incidence was 20%. After 7 days of follow-up, 35% of the controls were discharged, whereas 25% had died. One additional death occurred among the controls by day 14.
We report our experience using CP in the treatment of 20 severely and critically ill hospitalized COVID-19 patients and 20 matched controls. Although laboratory and respiratory parameters were improved in patients following CP infusion, their status was similar to that of controls. A similar proportion of patients in each group was discharged, whereas the 7- and 14- day fatality rate in CP patients compared favorably to that in controls. CP infusion was safe without adverse events. There was no evidence of clinical worsening to suggest a hyperimmune response.8 We did not see an increased risk for VTE in CP patients, although the incidence was high in both groups, despite heparin prophylaxis, as seen in COVID-19.9-11
In recent publications describing outcomes for hospitalized COVID-19 patients, mortality ranged between 26% and 88%.12-18 No deaths occurred in patients who received CP within 7 days of hospitalization, with the majority of patients receiving CP early. Findings from a prior study of patients with SARS-CoV-1 reported a survival benefit with early administration of CP (prior to 14 days after symptoms), as did a meta-analysis of studies conducted during Spanish influenza.19,20 These findings, along with a recent study reporting a lack of benefit when CP is administered later (median of 21.5 days after diagnosis) for SARS-CoV-2, support that earlier treatment may be of critical importance.21 Because viral loads peak in the first week for most viral infections, deterioration in the following weeks is thought to result from inflammatory destruction of lung tissue and would not be expected to improve with CP.19
Other than time to infusion, it is not clear how the volume of CP or neutralizing antibody titers of donors and/or recipients affect outcomes. In a prior study of CP for SARS-CoV-1, antibody titers and CP volumes did not appear to correlate with clinical response; however, patients who were positive via a quantitative reverse-transcriptase polymerase chain reaction assay and seronegative at the time of CP had a better outcome than did those who were already seropositive (66.7% vs 20%; P = .001).19 In 3 case reports of CP in SARS-CoV-2, viral loads became negative soon after CP, suggesting that antibodies from CP may contribute to viral clearance.5,6,21 However, additional factors in CP may be contributing to clinical improvement, because 4 of 10 patients reported by Duan et al had high neutralizing titers of 1:640 prior to CP.5
The limitations of this study included small sample size and short follow-up. Also, patients treated with CP and controls received additional therapies for COVID-19. The disparity in the use of RDV between the 2 groups is stark, with the CP group at 5% and controls at 50%. There are emerging data from the National Institutes of Health that RDV may result in a 31% improvement in time to recovery.22-24 If this effect was present in our control patients, then the outcome with CP may be even more remarkable.
In conclusion, the current study suggests that CP use in severe and critically ill patients with COVID-19 may improve survival if given early in the course of disease. The efficacy as a potential therapy needs further study in well-designed trials to better understand the contribution of CP to outcomes in COVID-19.
Data sharing requests should be sent to Livia Hegerova (livia.hegerova@swedish.org).
Acknowledgments
The authors thank all patients and their families for participating in the study. They also thank Mark H. Wener and Anu Chaudhary for helping to facilitate serologic testing.
This work was supported in part by research funding from Barbara and Kent Chaplin, a US Department of Health and Human Services (HHS), Biomedical Advanced Research and Development Authority (BARDA) grant contract 75A50120C00096, National Institutes of Health, National Center for Advancing Translational Seciences (NCATS) grant UL1TR002377, Schwab Charitable Fund (Eric E Schmidt, Wendy Schimdt donors), United Health Group, National Basketball Association (NBA), Millennium Pharmaceuticals, Octopharma Octapharma USA, Inc, and the Mayo Clinic.
Authorship
Contribution: L.H., N.B., M.B., V.D., K.P., J.M.P., T.A.G., K.A.S., and C.M. reviewed the literature, enrolled patients in the study, collected, analyzed, and interpreted data, and wrote the manuscript; K.A., R.H., J.M.J., and B.A.K. recruited donors and analyzed data; A.C.L. and M.L.A. collected data; J.D.G. was a member of the research advisory committee; and J.S.P., A.L., S.-j.L., and M.D.S. contributed control patient material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Livia Hegerova, Center for Blood Disorders and Stem Cell Transplantation, Swedish Cancer Institute, 1221 Madison St, Suite 1000, Seattle, WA 98104; e-mail: livia.hegerova@swedish.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal