Abstract
Anemia is a frequent complication of kidney disease. When severe, it causes symptoms that can be debilitating. The course of anemia tends to track the decline in kidney function, with prevalence increasing in more advanced disease. Although the most common cause is relative erythropoietin deficiency, other factors such as reduced iron availability contribute to the pathobiology. In this review, we use cases to explore the surprising complexity of decision-making in management of renal anemia.
Introduction
Anemia is a common complication of chronic kidney disease (CKD), first identified in 1836 by Sir Richard Bright who noted a fading of the “healthy colors of the countenance” among patients with kidney disease.1 In late kidney disease, anemia is a pervasive problem that can cause a variety of uncomfortable symptoms, making it one of the most important problems treated in CKD.
The prevalence and severity of anemia in CKD relate to the severity of CKD. We generally consider anemia as hemoglobin (Hb) <13 g/dL in men and <12 g/dL in women, with Hb <10 to 11 g/dL being more clinically relevant for intervention. Anemia in CKD is usually normocytic, normochromic, and hypoproliferative.2 We strongly recommend routinely estimating glomerular filtration rate (eGFR) using a stable serum creatinine and demographic data using an accepted equation.3 Patients with eGFR >60 mL/min/1.73 m2 must have accompanying persistent proteinuria or hematuria, or other evidence of chronic kidney injury to be considered CKD.4 Many do not carry a recognized CKD diagnosis because their serum creatinine is within the normal range. In CKD stage 1 and 2, which respectively have an eGFR >90 and 60 to 89 mL/min/1.73 m2, anemia is relatively uncommon and unlikely to be related to the underlying CKD.5
In stable outpatients with CKD stage 3a (eGFR, 45-59) and 3b (eGFR, 30-44), Hb <12 g/dL was reported in ∼42%, but Hb <10 g/dL was observed in only ∼6%.5 Most patients with CKD stage 3a and virtually all with stage 3b will have an elevated serum creatinine. In patients with stable CKD stage 4 (eGFR, 15-29), anemia was present in ∼54% whereas Hb was <10 g/dL in ∼11%. Approximately 75% of stage 5 nondialysis patients (eGFR, <15) have anemia, and ∼50% have Hb <10 g/dL. Among patients on dialysis, anemia is almost universal and severe enough that 90% require erythropoiesis-stimulating agent (ESA) treatment.6
Several factors contribute to causing anemia in CKD. A primary cause is relative erythropoietin (EPO) deficiency. In CKD, serum EPO concentrations are generally normal or slightly increased, but are inappropriately low for the degree of anemia7 (Figure 1). Other contributors to CKD-related anemia include uremic inhibitors, inflammation, shortened red blood cell survival, and nutritional deficiencies, such as vitamin B122 (Table 1).
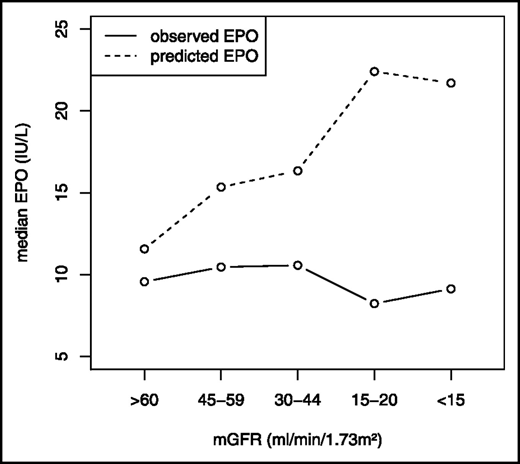
Relationship between serum EPO and mGFR. Mercadal et al7 studied the relationship between serum EPO and mGFR among anemic patients. They found that, as CKD progressed, the observed EPO response lagged well behind the expected response, indicating a relative deficiency of EPO. mGFR, measured glomerular filtration rate. Republished with permission of the American Society of Nephrology from Mercadal et al7 ; permission conveyed through Copyright Clearance Center, Inc.
Relationship between serum EPO and mGFR. Mercadal et al7 studied the relationship between serum EPO and mGFR among anemic patients. They found that, as CKD progressed, the observed EPO response lagged well behind the expected response, indicating a relative deficiency of EPO. mGFR, measured glomerular filtration rate. Republished with permission of the American Society of Nephrology from Mercadal et al7 ; permission conveyed through Copyright Clearance Center, Inc.
Common causes of renal anemia
| Decreased red cell production |
| Relative EPO deficiency |
| ID |
| Inflammation |
| Infection |
| Hyperparathyroidism |
| Nutritional deficiencies |
| Depletion of circulating red cell pool |
| Chronic blood loss, often occult |
| Reduced red cell circulating half-life |
| Frequent blood sampling |
| Decreased red cell production |
| Relative EPO deficiency |
| ID |
| Inflammation |
| Infection |
| Hyperparathyroidism |
| Nutritional deficiencies |
| Depletion of circulating red cell pool |
| Chronic blood loss, often occult |
| Reduced red cell circulating half-life |
| Frequent blood sampling |
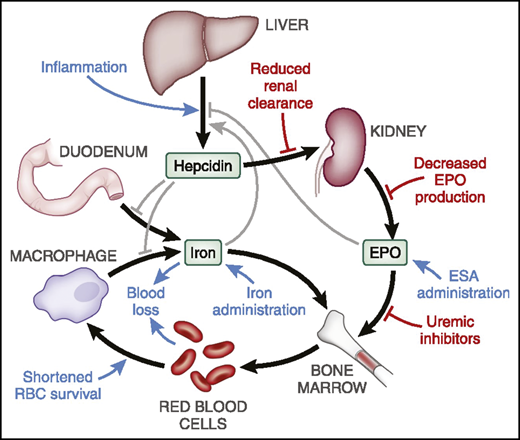
The role of inflammation is increasingly appreciated as a major factor in CKD-related anemia and complicates diagnosis of iron deficiency (ID).8,9 Inflammation results in increased hepatic production of the hAMP gene encoded protein, hepcidin,10 and increased serum ferritin, not only an indicator of iron storage but also another acute-phase reactant.11 Hepcidin is filtered and cleared by the kidney, and therefore CKD appears to contribute to higher hepcidin levels in CKD.12 Hepcidin blocks intestinal iron absorption and traps iron within reticuloendothelial system macrophages. The net effect is to limit the availability of iron for active erythropoiesis13 (Figure 2). Other contributors to anemia in CKD include increased intestinal blood loss and hyperparathyroidism. Consequently, the pathobiology of anemia in CKD is increasingly viewed as multifactorial.
Hepcidin plays a central role in iron metabolism and availability for erythropoiesis. When levels are elevated, intestinal iron absorption is diminished and release of stored reticuloendothelial system iron is blocked. The net effect is reduced iron availability for erythropoiesis. RBC, red blood cell. Republished with permission of the American Society of Nephrology from Babitt et al2 ; permission conveyed through Copyright Clearance Center, Inc.
Hepcidin plays a central role in iron metabolism and availability for erythropoiesis. When levels are elevated, intestinal iron absorption is diminished and release of stored reticuloendothelial system iron is blocked. The net effect is reduced iron availability for erythropoiesis. RBC, red blood cell. Republished with permission of the American Society of Nephrology from Babitt et al2 ; permission conveyed through Copyright Clearance Center, Inc.
Progressively increased hepcidin levels in CKD likely account for reduced iron availability being much more common in CKD patients than transferrin saturation (TSAT) or ferritin results might suggest. A study of 100 anemic CKD stage 4 and 5 patients who were ESA- and IV iron–naive found that 48% had absent iron stores on bone marrow aspiration. One-half of these iron-depleted patients had TSAT >20%, 52% had ferritin >100 ng/mL, and just 33% had TSAT <20% and ferritin <100 ng/mL.14 Given the difficulty in diagnosing ID using standard testing,15 empiric treatment with oral iron or 1 g of IV iron is often a therapeutic option.
Oral iron is the preferred route of administration in nondialysis CKD. Trials comparing oral iron to IV iron in stable CKD patients have found comparable improvements in Hb.16,17 If oral iron is not tolerated or a more rapid Hb response is needed, 1 to 1.5 g of IV iron are usually administered. Use of an ESA, such as epoetin, is considered for patients with Hb values <10 g/dL despite adequate iron stores or a trial of iron. In contrast, in hemodialysis, IV iron is usually required due to lack of efficacy of oral iron.18,19
Anemia of CKD has long been considered the cause of many symptoms that occur with diminishing renal function, including fatigue, decreased strength and stamina, and increased dyspnea with exertion. This is almost certainly true when severe anemia is present. Finkelstein et al found a strong association of higher Hb to several higher physical-functioning quality-of-life (QoL) parameters in patients with CKD stage 3, 4, and 5.20,21 Many people perceive that anemia causes fatigue, cold intolerance, and lack of stamina. Despite this general perception, randomized trial data confirming that these symptoms resolve or improve with anemia treatment have been relatively weak and absent, or have methodological issues such as lack of blinding.
The results of the double-blind TREAT study22 highlight the difficulty in attributing QoL changes to anemia rather than the inflammation and physical deterioration that accompany progressive CKD. The 4038-patient trial of type 2 patients with diabetes with CKD (eGFR, 20-60 mL/min/1.73 m2) and baseline Hb 9 to 11 g/dL compared treatment with darbepoetin to target Hb of 13 g/dL to placebo as long as Hb remained ≥9 g/dL. At 25 weeks, fatigue score was significantly improved in darbepoetin compared with placebo (Functional Assessment of Cancer Therapy [FACT]-Fatigue: mean change, 4.2 ± 10.5 vs 2.8 ± 10.3; P < .001). In the darbepoetin arm, 54.7% of had a minimally clinically significant 3-point improvement, vs 49.5% of the placebo arm.22 Although some attribute this small difference in QoL improvement to anemia treatment, it could also reflect inadvertent unblinding resulting in slight differences in patient reporting.4 A meta-analysis of randomized trials treating anemia in CKD to higher vs lower Hb targets with ESA found that higher Hb targets do not result in important differences in QoL.23
Although most studies have found that lower Hb levels are associated with worse patient outcomes, such as CKD progression, increased cardiovascular events, and death,24 randomized trials treating CKD-related anemia with ESAs have repeatedly shown that targeting higher Hb has increased cardiovascular events, heart failure (HF), thrombotic events including stroke, and death.22,25-27 Now, nephrologists generally accept that ESA therapy should not be initiated if Hb is >10 g/dL, and many defer ESA treatment until the Hb is below 9 g/dL. The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in Chronic Kidney Disease recommends starting ESAs using individualized decision-making.28 Factors influencing the decision to use ESA include history of strokes, functional status, transplant eligibility, and cancer history. These risks should be balanced against the need to avoid blood transfusions and the demonstrated value of ESA treatment in this regard.28
Patient 1
Anemia in a patient with CKD and macrocytosis
A 51-year-old man works full-time as a construction worker, and has had diabetes mellitus, hypertension, and CKD for >5 years. He has noted increasing fatigue, which parallels a slow decline in his renal function, and worsening anemia. His medications include an angiotensin-converting enzyme inhibitor, an sodium glucose cotransporter 2 inhibitor, and aspirin. His creatinine was 2.5 mg/dL (eGFR, 33 mL/min/1.73 m2), and he had normochromic, mildly macrocytic (mean cell volume, 102.2 fL) anemia with Hb of 9.3 g/dL despite 4 months of oral iron and an increase in his iron indices to TSAT of 24%, ferritin of 342 ng/mL. Tests of thyroid function, serum folate, and vitamin B12 were normal. His serum protein electrophoresis and immunofixation were negative, and his free light chain ratio showed mild elevations in κ and λ with a ratio of 1.65, consistent with CKD. The patient was started on epoetin alfa at 5000 U per week subcutaneously; over 2 months, his Hb rose to 10.4 g/dL.
Evaluation and treatment
The patient is anemic and has symptoms that might be related. In general, the new onset of fatigue in CKD should not be definitively attributed to anemia or CKD until other causes including HF, hypothyroidism, or depression have been excluded. In this case, no other cause for his fatigue was found.
This patient has several factors affecting his likelihood of developing anemia. Anemia is more common and more severe in patients with diabetes than nondiabetic, and those on angiotensin-converting enzyme inhibitors.29 Recently, sodium glucose cotransporter 2 inhibitors have been shown to increase Hb, at least in part by raising EPO levels.30 This patient’s history, examination, and laboratory tests do not point to a specific cause of anemia. One feature stood out as atypical for anemia in CKD, and that was the mild macrocytosis. The patient was on no medication that would cause macrocytosis and there was no history of alcoholism or liver disease from other causes; the thyroid testing was normal and there was no evidence for megaloblastic anemias (eg, B12 deficiency). Similarly, there was no evidence for other disorders of red cell maturation that could cause macrocytosis.
Because the diagnostic evaluation did not reveal other causes of anemia, EPO deficiency was considered the most likely etiology. Measurement of an EPO level is rarely indicated in CKD-related anemia. In patients without CKD, serum EPO and Hb are negatively correlated. In contrast, in patients with CKD stage 3 and 4, Mercadal et al found only a very weak negative correlation between Hb and EPO levels, and a failure of EPO levels to increase as Hb fell2,7 (Figure 2). Assays for EPO may detect inactive fragments, accounting for some of the poor correlation.2 Consequently, we virtually never order EPO levels in CKD-related anemia. The KDIGO anemia guidelines state that “erythropoietin levels are not routinely used in distinguishing EPO deficiency from other causes of anemia in patients with CKD in most clinical settings and their measurement is generally not recommended.”28(p290)
This patient should respond to ESA therapy within 2 to 3 months, as ∼90% of CKD patients have an adequate response. We would measure the Hb monthly, and adjust the epoetin dose to maintain his Hb between 10 and 11 g/dL. ESA treatment will draw down iron stores, and, therefore, we check TSAT and ferritin levels approximately every 6 months. If these tests suggest ID, or if the response to ESA fades, oral or IV iron is given. In our experience, we give IV iron to ESA-treated nondialysis CKD patients once every 12 to 24 months.
The US Food and Drug Administration (FDA) prescribing instructions for patients with nondialysis CKD state that “if the Hb level exceeds 10 g/dL, reduce or interrupt the (ESA) dose.”31 There does not appear to be evidence to support this management in CKD patients, and we find it unwieldy in practice, as there is always a delayed response to ESA after initiation. The FDA wording in theory creates payment barriers, but we have never had payors deny coverage when we started ESA when Hb was <10 g/dL and maintained Hb of 10 to 11 g/dL.
Based on the TREAT trial results,22 our patient’s only proven benefit will be a reduction in transfusions. This is particularly important among transplantation candidates whose alloimmune sensitization could delay or prevent receiving a transplant. Other risks factors for alloimmunization following transfusion include female sex especially if multiparous, other previous transfusions, and prior organ transplants.32
Given his relatively young age and full time manual labor, he may be more likely to report an improvement in his fatigue. Despite the trial results, improvements in functional status appear to be more common when the patient is more active and the anemia more severe. Some experts would consider targeting his Hb above 11 g/dL for a further improvement in QoL, although the trial evidence for this is weak or absent.32,33 Such management has the support of guidelines, though not evidence: “Individualization of (ESA) therapy will be necessary as some patients may have improvements in quality of life at Hb concentration above 11.5 g/dL and will be prepared to accept the risks.”28(p303) The clinician’s decision as to when a higher Hb target may be helpful should rely on factors such as younger age, greater functional status, physical activity, and freedom from risks related to cardiovascular disease. Use of IV iron may also contribute to an improvement in fatigue.
In addition to the inconvenience of a subcutaneous injection and monthly blood work, the risks of ESA therapy need to be weighed and conveyed to patients. Trials demonstrating the risks of ESA all targeted Hb >12 or 13 g/dL. As harm from ESA correlates with higher dose,34 and higher Hb targets require higher doses, we believe our present lower Hb goals mitigate some of the risks of ESAs. We also do not use the high ESA doses we did in the past. ESA dosing has decreased among US hemodialysis patients.35 In 2010, the mean and median ESA doses (in units per week) were 19 919 and 13 656, respectively; in 2019, they were 11 490 and 8693, respectively. Only 9% of patients receive >25 000 U per week presently, compared with 28% of patients in 2010.35
We routinely counsel patients that trials with ESA in CKD patients show an increased risk of cardiovascular events and death, increased strokes, and increased thrombotic events. In the TREAT trial, strokes were approximately doubled by ESA use, and in patients with a history of stroke, the risk of subsequent stroke was tripled.22 We counsel that lower ESA doses and lower Hb targets likely mitigate some of these risks. Many patients choose not to start an ESA when Hb is <10 g/dL, but as anemia worsens and the risk-benefit shifts, they agree to ESA therapy. Because anemia in nondialysis CKD is mild in the vast majority of patients, ESA use in practice occurs mostly in patients with later stage 4 CKD (eGFR, 15-20 mL/min/1.73 m2) or stage 5 CKD (eGFR, <15 mL/min/1.73 m2).
Patient 2
Anemia in a patient with CKD and HF
A 73-year-old man with hypertensive kidney disease and HF complains of shortness of breath and fatigue. His daily medications include 40 mg of lisinopril, 50 mg of metoprolol succinate, and 80 mg of furosemide. He has never been treated with an ESA or iron. There have been frequent adjustments to the diuretic dosing. His examination was notable for blood pressure of 138/64 mm Hg, crackles at the lung bases, a soft systolic heart murmur, and 1+ bilateral lower-extremity edema. Laboratory tests indicated normal electrolytes, serum creatinine of 2.9 mg/dL, eGFR of 23 mL/min, Hb of 10.8 g/dL with normal red cell indices, serum ferritin of 118 ng/mL, TSAT of 16%, and reticulocytes of 1.1%. The furosemide dose was increased to 80 mg in the morning and 40 mg in the afternoon and his symptoms improved; the serum creatinine and electrolytes remained stable.
Evaluation and treatment
There is a high rate of concordance of CKD and HF and each disease complicates the management of the other.36 The patient’s complaints may be due to CKD and HF, but the presence of anemia raises additional considerations. Certainly, the first priority is assessment of his current cardiac and volume status and adjustment of therapy to optimize each. In this case, after the diuretic dose was increased, there was only partial improvement of symptoms. Further decisions hinged on the severity of his cardiac disease and residual symptomatology. At this point, the contribution of anemia was considered as well.
Anemia is a frequent complication of HF independent of the presence of CKD.37 It is possible that some of this patient’s symptoms could have been referable to the anemia. The Hb concentration of 10.8 g/dL is not particularly low for his degree of CKD or HF, and most patients with an Hb level in this range would not be symptomatic. However, the relationship between symptomatology and Hb concentration is imprecise.
If the patient was to be treated, ESA therapy would not necessarily be an option. FDA prescribing instructions (label) do not support initiating ESAs when the Hb is above 10 g/dL.28,31 KDIGO guidelines, as we discuss later, are somewhat more flexible. Additionally, there is no FDA indication for the use of ESAs in HF.31
The cause of this patient’s anemia was likely multifactorial, including contribution from relative EPO deficiency, inflammation, dilution, and other factors. The initial laboratory testing suggests a contribution of ID as the serum ferritin is nondiagnostic and TSAT is low. Although neither test is particularly accurate for the diagnosis of ID in CKD, this patient’s laboratory results and the presence of HF strongly suggest he will have a favorable response to IV iron.14,38
Iron test results in CKD-related anemia are frequently nondiagnostic,15 often with an elevated ferritin, and a low or normal TSAT. Serum ferritin or TSAT are the most widely used tests in practice but lack accuracy, leading renal guidelines to state, “For adult CKD patients with anemia not on iron or ESA therapy we suggest a trial of IV iron (or in CKD ND patients alternatively a 1-3 month trial of oral iron therapy) if: an increase in Hb concentration without starting ESA treatment is desired* and TSAT is <30% and ferritin is <500 ng/mL.“28(p284) In other words, the trigger for treatment requires more than the test results due to their limited utility. Other tests, such as percent hypochromic red cells and reticulocyte Hb content, may offer greater accuracy for diagnosing ID, but are not routinely ordered; most nephrologists have limited experience with these tests. Consequently, although the British National Institute for Health and Care Excellence guidelines spoke to their potential role,11 their use is not widespread in nephrology clinical practice.
ID is common and strongly associated with worse outcomes in patients with HF.39-41 Observational studies of patients with HF report that 50% to 60% have ID (defined as ferritin <100 ng/mL regardless of TSAT, or ferritin 100-300 ng/mL and TSAT <20%), whereas ∼32% of HF patients have ID anemia.42,43 Compared with HF patients without ID or anemia, the hazard ratio (HR) for HF admission or death was 1.7 in anemic non-ID patients, 3.2 in nonanemic ID patients, and 4.9 in ID anemia patients42 Compared with controls, HF patients had decreased myocardial iron content and impaired mitochondrial function, possibly accounting for the worse outcomes.43
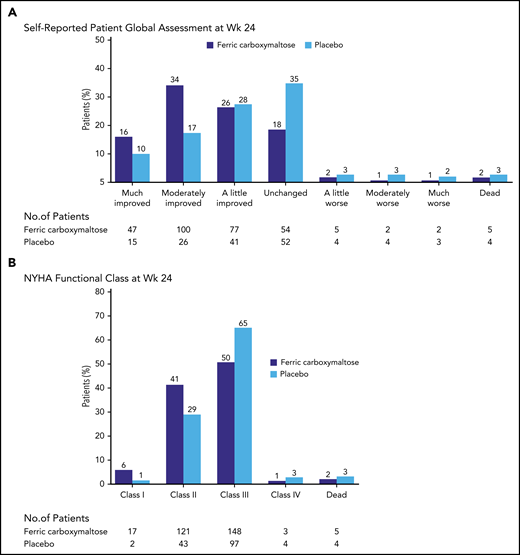
Consistent with this, a double-blind trial of IV iron vs placebo in 459 patients with HF due to reduced ejection fraction strongly favored use of IV iron (Figure 3). The primary end points were the self-reported Patient Global Assessment and New York Heart Association (NYHA) functional class at week 24. There were significant improvements in ID patients with or without anemia in symptoms, and in 6-minute-walk-test distance and QoL.38 A patient-level meta-analysis of 4 blinded trials of IV iron vs placebo in HF patients found that IV iron reduced recurrent hospitalizations and mortality (HR, 0.59), and recurrent HF hospitalizations and cardiovascular mortality (HR, 0.53).44
Study of HF and ID in 459 patients. Anker et al38 studied 459 patients with HF and ID. Patients were randomly assigned to be treated with 200 mg of IV ferric carboxymaltose or placebo. The primary end points displayed in this figure both demonstrated significant improvement with IV iron treatment. NYHA, New York Heart Association; Wk, week. From the New England Journal of Medicine, Anker SD et al, Ferric carboxymaltose in patients with heart failure and iron deficiency, volume 361, pages 2436-2448.38 Copyright © 2009 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Study of HF and ID in 459 patients. Anker et al38 studied 459 patients with HF and ID. Patients were randomly assigned to be treated with 200 mg of IV ferric carboxymaltose or placebo. The primary end points displayed in this figure both demonstrated significant improvement with IV iron treatment. NYHA, New York Heart Association; Wk, week. From the New England Journal of Medicine, Anker SD et al, Ferric carboxymaltose in patients with heart failure and iron deficiency, volume 361, pages 2436-2448.38 Copyright © 2009 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Together with the expected benefits of iron treatment in CKD, the positive effects in HF strongly support IV iron use in our patient. The expected benefits of IV iron in our patients should be contrasted with the results of ESA therapy in the HF population. A double-blind trial of darbepoetin vs placebo found no reduction in all-cause death or hospitalization for HF (HR, 1.01), whereas thromboembolic events were more common with the ESA (P = .01).45 Although adverse outcomes were more common in patients who were hyporesponsive to ESA, it was difficult to identify these patients using clinical or biochemical markers.46
Patient 3
A 38-year-old nulliparous white woman on chronic hemodialysis (HD) for 4 years has recently been diagnosed with breast cancer. She has been receiving 2200 U of epoetin thrice weekly in HD and 50 mg of IV iron sucrose weekly; her Hb range was 10.5 to 11.2 g/dL. In addition to addressing whether she should continue on an ESA, consideration was given to the patient’s desire for a kidney transplant although she had heard that transfusions could reduce her chances of receiving one.
Cancer among patients on chronic dialysis is common. Approximately 25% have a history of malignant neoplasm reported at initiation of dialysis, and the 5-year cumulative incidence of a new cancer is estimated at ∼10%.47 Although it is unclear whether the use of ESA may lead to progression of some cancers,31,48 and the FDA label recommends against use of an ESA when the expectation for treatment is cure,31 we frequently need to reassess the use of ESA in the dialysis patient.
Hemodialysis facilities have protocols to initiate and adjust the ESA dose based on recent Hb trends, and almost all US patients are managed using these protocols. Dose changes are typically ∼25% up or down every 4 weeks, to maintain Hb of 10 to 11 g/dL. Protocols for IV iron vary, but usually specify small weekly or biweekly doses to maintain TSAT >20% to 25% and ferritin below a specific limit of usually 800 or 1200 ng/mL. Patients with low TSAT and ferritin may receive a loading dose of iron as 100 mg every treatment of 10 doses. Among US hemodialysis patients in 2019, the estimated median Hb was 10.7 g/dL, the epoetin dose was 7900 U per week, and the monthly IV iron dose was 218 mg; the mean ferritin was 815 ng/mL and TSAT was 30%.35
Regular-maintenance iron reduces ESA requirements, and, in some, eliminates the need for ESA therapy. However, repeated concerns have been raised about the safety of IV iron due to observational data associating higher doses with cardiovascular events, death, and infections.49 Recently, a 4-year randomized trial compared proactive IV iron use (400 mg of IV iron monthly if ferritin <700 ng/mL and TSAT <40%) vs conservative IV iron (100 to 200 mg iron monthly if ferritin <200 ng/mL or TSAT <20%). The primary end point of cardiovascular events and death was significantly lower in patients given proactive IV iron.50 Additionally, proactive iron use led to fewer transfusions, an ∼20% lower ESA dose, and no increase in infections.50
Although this study considerably diminishes previous concerns, it does not fully eliminate unease as to IV iron safety. It can reasonably be concluded that higher dose compared with lower dose IV iron appears to have been safe. But both groups were treated with a significant amount of IV iron, and there was no group that did not receive IV iron, so there remains some residual uncertainty as to safety. It remains possible that a group that did not receive any IV iron might have had, for example, fewer infections.50 The study does not inform with regard to one of the most common questions in IV iron therapy: how high should serum ferritin be allowed to rise during therapy? No evidence supports any specific answer to this question. As for all treatment, risks and benefits should be weighed prior to and during treatment. In particular, IV iron should probably not be administered during an episode of infection.
Although ∼90% of HD patients receive an ESA in any 3-month period, not all develop severe anemia if ESA therapy is withheld. This patient has a low epoetin dose, and relatively high Hb. Prior to the ESA era in 1990, the mean Hb in HD patients was ∼9 g/dL, and ∼15% of patients received at least 1 transfusion per calendar quarter.51 Thus, stopping the ESA may not result in severe anemia. The cancer management choices may also induce worsening anemia despite ESA therapy. Transfusions can lead to antibody formation, particularly in multiparous women, and lead to difficulty finding an acceptable kidney transplant after the cancer is cured.32 Lastly, most hemodialysis patients are not transplant candidates or have chosen not to pursue transplant.
Our general approach is to discuss the relevant issues with the patient and oncologist. We ask oncologists about the likelihood of cure and goals of therapy, their perception of ESA adversely affecting the cancer outcome, and expected cancer treatment and its likelihood of inducing severe anemia or requiring transfusions. We review the ESA product information with patients, listen to the their concerns, and ascertain whether they are willing to continue on an ESA. In addition to ESA treatment, other tools that can improve Hb concentration in these patients include: avoiding use of dialysis catheters as they increase inflammation, blood loss, and risk of infection; controlling hyperparathyroidism; and modifying the dialysis prescription.
In many cases, we initially withhold the ESA, continue maintenance IV iron, and monitor Hb twice monthly with a goal of maintaining Hgb >9 g/dL. If Hgb falls below this threshold, we reassess the patient’s fatigue, and reweigh the risks of benefits of ESA use. If Hb is persistently <8 g/dL, we usually will favor use of low doses of epoetin (<10 000 weekly) to maintain Hgb >9 g/dL, provided the patient and oncologist are agreeable.
Authorship
Contribution: S.F. and D.W.C. designed and wrote the manuscript.
Conflict-of-interest disclosure: S.F. received research funding from AstraZeneca, Akebia, Megapro, and Corvidia, and provided consulting services to AstraZeneca, Rockwell, and Corvidia. D.W.C. provided consulting services to Fresenius, Fibrogen, GlaxoSmithKline, and AstraZeneca.
Correspondence: Steven Fishbane, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, 100 Community Dr, Great Neck, NY 11021; e-mail: sfishbane@northwell.edu.
REFERENCES
Author notes
S.F. and D.W.C. contributed equally in all aspects of design and writing.