Introduction
A direct association exists between minimal residual disease (MRD) negativity and prolonged survival in multiple myeloma (MM) (Landgren et al, BMT 2016). 18F-fluoro-deoxy-glucose (FDG) positron emission tomography-computed tomography (PET/CT) is a recommended monitoring technique for patients with MM as persistence of FDG uptake after induction therapy, prior to maintenance, is an independent risk factor for progression. Therefore PET/CT and MRD detection in the bone marrow are complementary prognostic tools prior to initiation of maintenance therapy. In patients with smoldering multiple myeloma (SMM), the presence of a focal FDG-avid lesion without underlying osteolytic lesion on PET/CT is associated with rapid progression to MM. However, little is known about the prognostic value of PET/CT for SMM patients receiving treatment. Herein, we show that treatment of high risk (HR)-SMM with carfilzomib, lenalidomide, and dexamethasone with lenalidomide maintenance (KRd-R) leads to sustained remissions detected on PET/CT imaging.
Methods
Trial design including key results for KRd-R in HR-SMM (NCT01572480) has been submitted to the meeting separately (abstract ID: 136148). As part of the study design, all eligible patients had bone marrow biopsies with multicolor flow cytometry (MRD sensitivity, 10-5) and whole-body PET/CT performed at baseline and at key time points, including achievement of complete response (CR) or completion of KRd induction (8 cycles), after 1 and 2 years of -R maintenance, and annually thereafter. PET/CTs were evaluated by nuclear medicine radiologists blinded to flow cytometry and considered positive if at least one focal hypermetabolic (above background reference) lesion and/or heterogenous bone marrow involvement were present, as defined by the IMWG (Hillengass et al. Lancet Oncol 2019).
Results
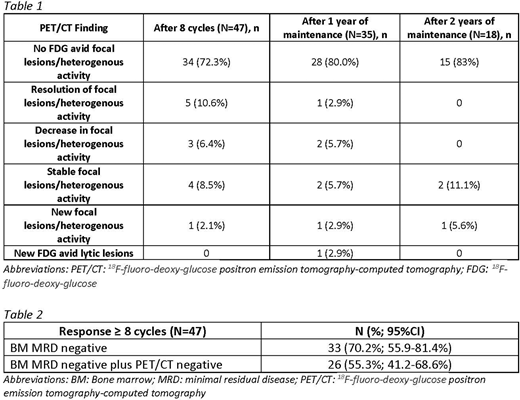
As of data cutoff, 46 patients had completed at least 8 cycles of therapy and had 2 sequential PET/CTs performed. By the end of induction therapy, no patient developed progressive disease and the overall response rate was 100%. Approximately 72% of patients with baseline negative PET/CTs remained negative, 11% of patients had resolution of previous focal/heterogenous FDG avidity, 15% of patients had decrease or stable focal/ heterogenous lesions, and 2% developed new focal lesions. Table 1 shows the results at subsequent time points of one and two years of maintenance therapy. Throughout this time period, one patient developed a lytic lesion after 1 year of maintenance therapy. However, 3 patients had either resolution or decrease in focal/heterogenous lesions. Specifically, after 8 cycles of combination therapy, 33 patients (70.2%, 95% CI 55.9 - 81.4%) had a response of MRD negative CR based on bone marrow flow cytometry and 26 patients (55.3%; 95% CI 41.2-68.6%) had a negative PET/CT in addition to MRD negative CR (Table 2).
Conclusions
It is important to evaluate the tools used in MM response assessment specifically in the SMM population as more studies report results of treatment in this population. MRD information can be used as a biomarker to evaluate the efficacy of different treatment strategies. This study demonstrates an exceptionally high rate of concordance between MRD negativity by flow cytometry and negative PET/CT after 8 cycles of KRd. However, 15% of patients were MRD negative yet had positive findings on PET/CT. While these lesions were not biopsy proven, some resolved during maintenance therapy. Further follow-up is needed to determine whether early MRD negativity in bone marrow with negative PET/CT correlates to longer overall survival and decreased progression to MM compared to those patients with a positive PET/CT. The use of PET/CT imaging may increase our understanding in assessing depth response to treatment in HR-SMM patients and be an important outcome predictor.
Korde:Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Landgren:Adaptive: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Takeda: Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Glenmark: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Research Funding; Janssen: Consultancy, Honoraria, Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Karyopharma: Research Funding; Binding Site: Consultancy, Honoraria; Takeda: Other: Independent Data Monitoring Committees for clinical trials, Research Funding; BMS: Consultancy, Honoraria; Cellectis: Consultancy, Honoraria; Glenmark: Consultancy, Honoraria, Research Funding; Juno: Consultancy, Honoraria; Seattle Genetics: Research Funding; Pfizer: Consultancy, Honoraria; Merck: Other; Karyopharma: Research Funding; Binding Site: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Cellectis: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Merck: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal