Background- In patients with light chain amyloidosis (AL), t(11;14) detected by fluorescence in situ hybridization (FISH) is the most common cytogenetic aberration. Several studies have shown that t(11;14) is associated with inferior outcomes in newly diagnosed AL patients [1, 2]. In contrast, at least one study in patients with t(11;14) who underwent high-dose therapy and autologous hematopoietic stem cell transplantation (auto-HCT) showed improved complete response (CR) rate and prolonged hematologic event-free survival[3]. In this single-center, retrospective analysis, we evaluated the outcome of patients with AL and t(11;14) who underwent auto-HCT at our institution.
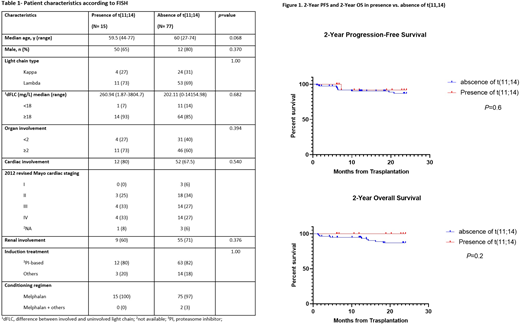
Method- We identified 122 consecutive patients with AL with cardiac or renal involvement who received an auto-HCT between 2011 and 2019. Baseline FISH data were available for 92 patients, 15 (16 %) of whom had t(11;14). Seventy-seven (84%) patients without t(11;14) were included as control . Hematologic and organ responses were evaluated according to the Consensus Guidelines for AL [4]. Revised Mayo staging system was utilized for Cardiac staging [5].
Result- The median age at auto-HCT was 60 years (range, 27 to 77). There were no significant differences in baseline characteristics between the two groups (Table 1). The median follow-up from auto-HCT was 28 months (range, 1 to 100). Overall, 40%, and 42% of patients with or without t(11;14), respectively (p=0.573), received post-auto-HCT maintenance therapy. One-year non-relapse mortality (NRM) was 2%. The 1-year NRM was 0 and 2.6% (n=2) in patients with or without t(11;14) (p=0.366). Hematologic CR after auto-HCT was seen in 7 (47%) and 33 (42%) patients with or without t(11;14), respectively (p=0.78). Organ response (OR) after auto-HCT was seen in 10 (71%) and 50 (67%) patients with or without t(11;14), respectively (p=0.586). The 2-year hematologic disease-free survival (Heme DFS) was 93% and 87% with or without t(11;14), respectively (p=0.422). The 2-year progression-free survival (PFS) was 92%, and 87% in patients with or without t(11;14) (p=0.6) (Figure 1A).The 2-year overall survival was 100%, ad 87% in patients with or without t(11;14) (p=0.2) (Figure 1B). Cardiac involvement with AL was associated with a shorter OS (p=0.012).
Conclusion- In this single-center retrospective analysis, we showed that auto-HCT is safe and feasible in selected patients with AL and t(11;14), and these patients have comparable outcomes to patients without t(11;14).
Bashir:Celgene: Research Funding; StemLine: Research Funding; Acrotech: Research Funding; Takeda: Other: Advisory Board, Research Funding; KITE: Other: Advisory Board; Purdue: Other: Advisory Board; Amgen: Other: Advisory Board. Hosing:NKARTA Inc.: Consultancy. Popat:Bayer: Research Funding; Novartis: Research Funding. Kebriaei:Amgen: Other: Research Support; Pfizer: Other: Served on advisory board; Kite: Other: Served on advisory board; Novartis: Other: Served on advisory board; Ziopharm: Other: Research Support; Jazz: Consultancy. Shpall:Takeda: Other: Licensing Agreement; Magenta: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Zelluna: Membership on an entity's Board of Directors or advisory committees. Manasanch:Adaptive Biotechnologies: Honoraria; Sanofi: Research Funding; Novartis: Research Funding; JW Pharma: Research Funding; Merck: Research Funding; Quest Diagnostics: Research Funding; Takeda: Honoraria; BMS: Honoraria; Sanofi: Honoraria; GSK: Honoraria. Lee:Amgen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Genentech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Daiichi Sankyo: Research Funding; Regeneron: Research Funding; Genentech: Consultancy. Kaufman:Janssen: Research Funding; Karyopharm: Honoraria; Bristol Myers Squibb: Research Funding. Patel:Oncopeptides: Consultancy; Celgene: Consultancy, Research Funding; Cellectis: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Precision Biosciences: Research Funding; Poseida: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Thomas:Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; X4 Pharma: Research Funding; Xencor: Research Funding; Pharmacyclics: Other: Advisory Boards; Genentech: Research Funding; BMS: Research Funding. Orlowski:STATinMED Research: Consultancy; Founder of Asylia Therapeutics, Inc., with associated patents and an equity interest, though this technology does not bear on the current submission.: Current equity holder in private company, Patents & Royalties; Sanofi-Aventis, Servier, Takeda Pharmaceuticals North America, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen, Inc., AstraZeneca, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc., Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, Inc.,: Honoraria, Membership on an entity's Board of Directors or advisory committees; Laboratory research funding from BioTheryX, and clinical research funding from CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding. Champlin:Actinium: Consultancy; Omeros: Consultancy; Takeda: Patents & Royalties; Cytonus: Consultancy; DKMS America: Membership on an entity's Board of Directors or advisory committees; Genzyme: Speakers Bureau; Johnson and Johnson: Consultancy. Qazilbash:Janssen: Research Funding; Bioline: Research Funding; Angiocrine: Research Funding; Amgen: Research Funding; Bioclinica: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal