Background: High fetal hemoglobin (HbF) levels reduce mortality and morbidity in sickle cell disease (SCD). Results from a prior clinical trial to assess safety of high dose vitamin D and our unbiased genomic analyses identifying a vitamin D regulated protein as a HbF inducer suggests that vitamin D replacement may increase HbF levels in SCD patients. To test this hypothesis, we performed a single center retrospective chart review investigating the impact of vitamin D replacement on HbF levels in our pediatric SCD population.
Methods: We reviewed electronic medical records of pediatric patients with SCD with one or more serum vitamin D level who used vitamin D replacement from January 2007 to March 2020. SCD patients on chronic blood transfusion were excluded. Our cohort contained 81 SCD patients (ages: 2.3-19.9 years; 40 males) 71 HbSS, 6 HbSC, 3 HbSβ0, and1 Hb S-δβ. Vitamin D deficiency was defined as vitamin D levels < 30 ng/ml. All subjects were on hydroxyurea, with stable MCV (mean 96 fL at baseline and 95.6 fL during the study period). The patients received a median vitamin D dose of 2002 IU/day for a median of 229 days with a median follow-up of 365 days after replacement.
Statistical analyses: Vitamin D deficient and non-deficient SCD patients data was compared at baseline by t test (for continuous variables with normal distribution), Wilcoxon test (for continuous variables with non-normal distribution), chi2 test (for categorical variables), and Fisher exact test (for categorical variables with small sample size). Linear relationships between vitamin D levels and HbF, CBC, BMI, and age were assessed using scatter plots and correlation coefficients (r value). Non-normal variables were log transformed to achieve normal distribution. A linear mixed effect (MLE) models were run to analyze linear relationship of variables of time dependent variables, including variable number of clinic visits and variable follow-up time. All models had 'vitamin D level" as an exposure variable; hydroxyurea and folic acid use as covariates, and one of the following as an outcome variable: HbF, hemoglobin, MCV, MCHC, reticulocyte count, WBC count, and platelet count. We performed mediation analysis to determine if HbF or MCV were intermediate variables for the effect of vitamin D on reticulocyte count. All LME models and scatter plots were created separately for the period of vitamin D replacement and a one-year follow-up period after cessation of vitamin D replacement to capture any lag period in HbF induction and sustained effect of vitamin D replacement.
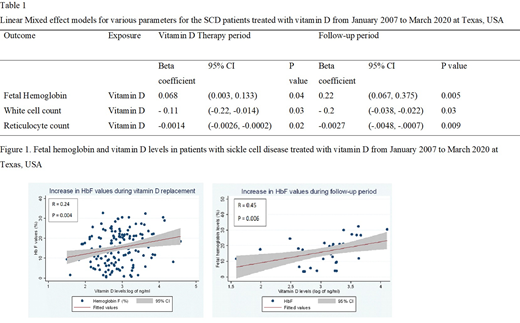
Results: The LME model, adjusting for hydroxyurea and folic acid, indicated that HbF increased by 0.68 percentage points with every 10 ng/ml rise in vitamin D levels during the vitamin D replacement period (p=0.04) and continued to rise after replacement, exhibiting a 2.2 percentage point rise per 10 ng/mL increase above replacement HbF levels during a 12 month follow-up period (p=0.005) (Figure 1, Table 1). WBC and absolute reticulocyte count (ARC) decreased significantly following vitamin D replacement according to the LME models (p<0.05) (Table 1). Reduction in ARC was due to HbF induction as per the mediation analysis.
Conclusion: SCD patients have a 56.4-96.4 % prevalence of vitamin D deficiency reported in the literature. Several studies have shown that vitamin D deficiency is associated with increased morbidity and mortality in SCD patients. However, vitamin D screening and replacement are not routinely implemented in clinical practice. Our study is the first to analyze the effect of vitamin D replacement on HbF levels as a primary objective using longitudinal panel data. Our findings strongly suggest that vitamin D replacement can increase HbF levels and reduce laboratory markers such as ARC and WBC, whose elevation is associated with clinical severity.
Sheehan:Emmaus: Research Funding; Novartis: Research Funding; Global Blood Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal