Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare systemic thrombotic microangiopathy (TMA) characterized by hemolytic anemia, thrombocytopenia and end organ damage, predominately in the kidneys. Disease emergence is often unpredictable, occurring with or without an identified trigger or in the presence of a concomitant clinical condition. The persistence of TMA and long-term disease manifestations are also not well described. The aims of this study were to characterize the frequency and distribution of potential triggering conditions preceding a diagnosis of aHUS, and to describe the persistence of TMA and long-term disease manifestations in those not treated with eculizumab.
Methods
This retrospective study included two cohorts of patients with aHUS identified in the Optum® Clinformatics® Data Mart commercial and the Medicare Advantage claims databases, encompassing approximately 62 million patients, from January 2007 to March 2019. As aHUS does not have a dedicated diagnosis code, patients with aHUS were defined as having ≥ 2 HUS or TMA diagnostic codes, and ≥ 1 claim for each of the following aHUS-associated clinical manifestations: hemolytic anemia, thrombocytopenia, and renal/neurological/gastrointestinal organ involvement. Patients with paroxysmal nocturnal hemoglobinuria, myasthenia gravis or Shiga toxin-producing E. coli diagnoses were excluded. Additionally, patients were required to have ≥ 1 lactate dehydrogenase, reticulocyte, schistocyte, haptoglobin or blood smear test within 3 months of any HUS or TMA diagnosis, or ≥ 1 ADAMTS13 test with results demonstrating activity of ≥ 5%. Eculizumab, a C5 inhibitor, was the only approved therapy during this time period.
Two cohorts were analyzed. Cohort 1: eligible patients with aHUS were required to have ≥ 12 months continuous database enrollment prior to and ≥ 18 months after the date of first HUS or TMA diagnostic code, and may or may not have been receiving eculizumab. Clinical disease manifestations were evaluated up to 15 months after the date of first aHUS diagnosis. Cohort 2: patients with aHUS and persistent TMA (≥ 1 International Classification of Diseases code for TMA and one diagnosis of thrombocytopenia within 12 months following the first TMA diagnosis) were identified. Eligible patients were required to have 3 months enrollment prior to and 12 months after the date of first TMA diagnosis. Those receiving eculizumab were excluded. Claims data relating to prespecified potential triggers of aHUS were identified during the 0-3-month period prior to the first diagnosis of HUS or TMA. Data were summarized using descriptive statistics.
Results
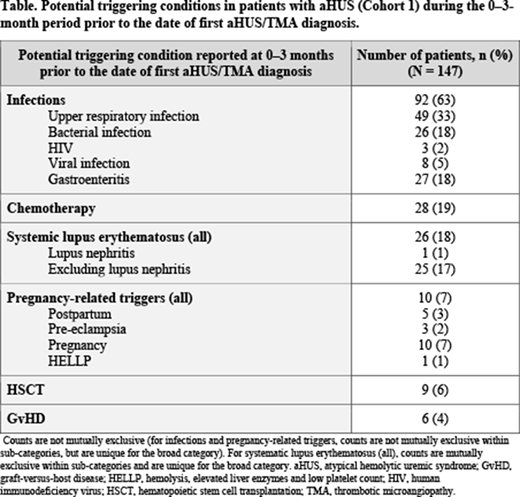
Cohort 1: a total of 431 patients with aHUS were identified (mean age of 58 years, 55% female). Prespecified triggers for the development of aHUS were identified in 147 patients (34%), with infections representing the most common trigger (reported by 92 patients [63%]), followed by chemotherapy (28 patients [19%]) and systemic lupus erythematosus (26 patients [18%]), as shown in the Table. Among patients not receiving eculizumab (n = 415), disease manifestations persisted for > 1 year following the first diagnosis date of aHUS, with 298 patients (72%) reporting clinical disease manifestations 13-15 months after diagnosis. Kidney disease represented the most common manifestation (90 patients [30%] with chronic kidney disease and 26 patients [9%] with end-stage renal disease), followed by thrombocytopenia (87 patients [29%]) and hemolytic anemia (82 patients [28%]). Cohort 2: among those with aHUS and persistent TMA (n = 218; 69.3% of patients aged 35-74 years, 67% female), a total of 191 patients had data available for analysis. Of these, 95 patients (50%) reported prespecified triggers, with a similar trigger pattern observed as in the patients with aHUS in Cohort 1.
Conclusions
Triggers of aHUS were identified in 34% of patients with aHUS and 50% of those with aHUS and persistent TMA. In those with aHUS alone not receiving eculizumab, long-term clinical disease manifestations persisted in the majority of patients. Further evidence on treating trigger-associated aHUS with complement inhibition is required.
Tomazos:Alexion Pharmaceuticals Inc.: Current Employment. Garlo:Alexion Pharmaceuticals Inc.: Current Employment. Wang:Alexion Pharmaceuticals Inc.: Current Employment. Chen:Alexion Pharmaceuticals Inc.: Current Employment. Laurence:Alexion Pharmaceuticals: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal