Background: Ibrutinib (Ibr) is the only once-daily Bruton's tyrosine kinase (BTK) inhibitor with significant survival benefit vs chemo- and/or immunotherapy in multiple phase 3 studies of patients (pts) with CLL. Ibr in first-line (1L) CLL is associated with lower rates of progressive disease (PD) than in later lines. Acquired mutations in BTK or phospholipase C-γ2 (PLCG2) genes have been shown in ~70-80% of pts with CLL progression on ibr or acalabrutinib. Most studies reporting BTK/PLCG2 mutation rates to date have been conducted in pts with PD. The objective of this study was to systematically evaluate the frequency and time to detection of BTK and PLCG2 mutations in pts who were continuing to respond to ibr (free of PD) in either 1L or R/R treatment.
Methods: Peripheral blood samples were prospectively collected from clinical trial pts treated with ibr in 1L (RESONATE-2, iLLUMINATE, and NCT01500733) or R/R settings (RESONATE and RESONATE-17). After CD19 enrichment, established BTK and PLCG2 mutation testing was performed by next-generation sequencing at the last available sample timepoint for pts without PD. Mutation frequencies are summarized using descriptive statistics. For pts with identified BTK mutations, earlier timepoints were assessed by C481x-specific droplet-digital PCR. Prespecified Kaplan-Meier analyses were used to characterize time from ibr initiation to first detection of BTK mutations and to assess differences between specified populations, including 1L or R/R treatment; presence or absence of del(17p)/TP53; and 1 or ≥3 prior therapies.
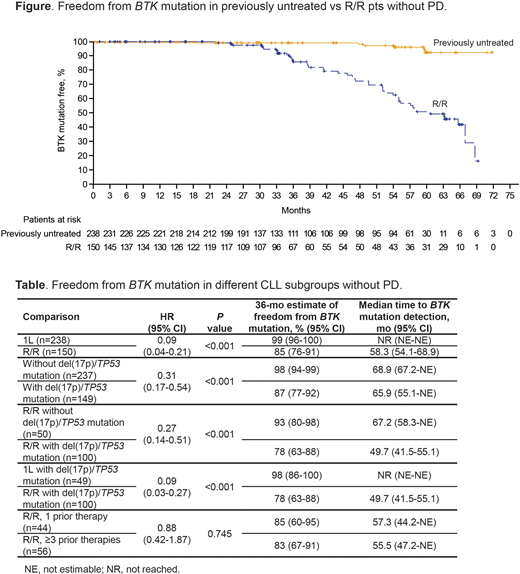
Results: Of 388 pts tested, 238 (61%) were previously untreated and 150 (39%) had R/R disease (del[17p]/TP53 mutations in 49 and 100 pts, respectively). With median testing follow-up on treatment of 35 mo (range 0-72) and 36 mo (range 1-69), the BTK mutation rate was 3% and 30% in previously untreated and R/R pts, respectively. In the 1L and R/R settings the rates of PLCG2 mutations were 2% and 7%, and the rate of co-occurring BTK/PLCG2 mutations were 1% and 5%, respectively. Of the prespecified comparisons, there was superior freedom from detection of BTK mutations (Table) in pts treated with ibr in the 1L vs R/R setting (hazard ratio [HR] 0.09; P<0.001; median time to detection: not reached vs 58 mo; 36-mo mutation-free estimates: 99% vs 85%) (Figure); pts in the overall population without vs with del(17p)/TP53 mutations (HR 0.31; P<0.001; 36-mo mutation-free estimates: 98% vs 87%); and R/R pts without vs with del(17p)/TP53 mutations (HR 0.27; P<0.001; 36-mo mutation-free estimates: 93% vs 78%). Among all pts with del(17p)/TP53 mutations, improved freedom from BTK mutation was seen in previously untreated vs R/R pts (HR 0.09; P<0.001; 36-mo mutation-free estimates: 98% vs 78%). Among R/R pts, although a lower frequency of mutations occurred in those with 1 vs ≥3 prior lines of therapy (23% vs 39%), there was no difference in freedom from BTK mutation between the 2 groups when analyzed by Kaplan-Meier methods (HR 0.88; P=0.745; 36-mo mutation-free estimates: 85% vs 83%). Further analyses revealed that line of therapy in R/R pts predicts mutation rates only in the presence of del(17p)/TP53 mutations. In pts with del(17p)/TP53 mutations, the 1 prior line group had lower mutation rates than the ≥3 prior therapies group (18% [6/33] and 49% [17/35]), whereas mutation rates were similar for both in the absence of del(17p)/TP53 mutations (36% [4/11] and 33% [7/21]).
Conclusions: In this large study of 388 pts with diverse clinical risk factors and up to 6 years of follow-up, BTK and PLCG2 mutation rates were rare for pts on 1L ibr treatment, consistent with the low frequency of PD in this setting. Detection of BTK mutations was more common in R/R than previously untreated pts (30% vs 3%). These data in pts without PD and with this long-term follow-up are consistent with consensus guidelines, which recommend that the detection of BTK or PLCG2 mutations does not currently warrant changes in the treatment of pts with CLL. The presence of del(17p)/TP53 increases the risk of mutation development both overall and in R/R pts and is more informative than number of prior lines of therapy. Ongoing analyses will further characterize the time of first detection of BTK and PLCG2 mutations, change in allele frequencies over time, relationship between timing and kinetics of mutations and development of PD, and populations that are at relatively increased risk for mutation development.
Wiestner:Pharmacyclics LLC, an AbbVie Company, Acerta, Merck, Nurix, Verastem, and Genmab: Research Funding; NIH: Patents & Royalties: NIH. Ghia:Adaptive, Dynamo: Consultancy, Honoraria; Celgene/Juno: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; BeiGene: Consultancy, Honoraria; Acerta/AstraZeneca: Consultancy, Honoraria; ArQule: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; MEI: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Novartis: Research Funding; Sunesis: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding. Byrd:Acerta Pharma: Research Funding; Syndax: Research Funding; Trillium: Research Funding; Kartos Therapeutics: Research Funding; Leukemia and Lymphoma Society: Other; Janssen: Consultancy; Vincera: Research Funding; Novartis: Research Funding; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, BeiGene: Research Funding; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, Novartis, Janssen: Speakers Bureau; Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Gilead, TG Therapeutics: Other. Moreno:Janssen: Speakers Bureau; AbbVie and Janssen: Research Funding; Janssen, AbbVie, Sunesis, and AstraZeneca: Consultancy. O'Brien:Kite, Regeneron, Acerta: Research Funding; Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc. Vaniam Group, AbbVie, Alexion, Verastem, Eisai, Juno Therapeutics, Vida Ventures: Consultancy; Gilead, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis: Consultancy, Research Funding. Jones:Pharmacyclics LLC, an AbbVie Company: Patents & Royalties: and other intellectual property, Research Funding. Cheung:Pharmacyclics LLC, an AbbVie Company: Current Employment, Patents & Royalties: and other intellectual property; AbbVie and Eli Lilly: Current equity holder in publicly-traded company. Chong:Pharmacyclics LLC, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Kwei:AbbVie and Gilead: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Dean:Pharmacyclics LLC, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. James:AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment, Other: Leadership, Patents & Royalties: and other intellectual property. Woyach:Pharmacyclics, Janssen, Morphosys, Karyopharm, Verastem, Abbvie, Lox: Research Funding; Pharmacyclics LLC, an AbbVie Company, AbbVie, Janssen, AstraZeneca, ArQule: Honoraria; Janssen, Pharmacyclics, AstraZeneca, Abbvie, Arqule: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal