
Background: Lower gastrointestinal (LGI) tract acute GVHD occurs in ~30% of patients after allogeneic hematopoietic cell transplantation (HCT). Failure to respond to first line treatment with corticosteroids (steroid-refractory (SR)-GVHD) has poor prognosis, with an overall response rate (ORR) of less than 50% and complete response (CR) rate <30% with subsequent therapies. Vedolizumab is a humanized monoclonal antibody that inhibits lymphocyte trafficking to gut by blocking the interaction of α4β7 on T cells with MAdCAM-1 on endothelium of venules within the GI tract. The role of vedolizumab for treatment of SR-LGI GVHD was assessed in a retrospective study of 25 patients evaluable for response who were treated at 7 sites [Floisand; BBMT 2019]. The median time to start of vedolizumab from HCT was 36 days, and 14 days from the diagnosis of LGI GVHD. The ORR at 6-10 weeks was 64% and 6 months overall survival (OS) was 54%. Herein, we summarize the MD Anderson experience with Vedolizumab in 20 patients with SR-LGI GVHD.
Methods
Patients ≥ 18 years with SR-LGI GVHD who were treated with vedolizumab between 03/2016 and 10/2019 were included. Other criteria were absence of liver GVHD, Karnofsky performance >30, any donor/graft and any conditioning. SR-GVHD was defined as failure to respond after 7 days of treatment with prednisone 2 mg/kg/day (or methylprednisolone equivalent), progression after 72 hours, or flare with steroid taper. Objectives were to determine LGI aGVHD response at day 14, 28 and 56 from start of vedolizumab, OS and non-relapse mortality at 6 month and toxicities from vedolizumab (any infection within 6 months of the last dose, hepatotoxicity, progressive multifocal leukoencephalopathy (PML) or infusion reactions). Vedolizumab 300 mg IV was given at 0, 2 and 6 weeks, and then every 8 weeks depending upon response. Acute GVHD was staged and graded as per the consensus criteria, and the standard definitions were used to assess response.
Results: Median age was 46 years (range, 23-71). Majority (75%, n=15) received peripheral blood graft; 35% (n=7) had myeloablative conditioning. Two patients had prior allogeneic HCT. Donor was MUD (35%), MSD (30%), haplo (10%), cord (20%) or MMUD (5%). GVHD prophylaxis was PTCy/MMF +/-tacro (40%), tacro/MMF +/-ATG (35%) or tacro/methotrexate +/-ATG (25%). All but 2 patients (90%) had grade 3-4 GVHD (45% stage 4, 40% stage 3 LGI GVHD) at the time of vedolizumab. Median time to start of vedolizumab after LGI GVHD diagnosis was 21 days (range, 5-1031), and 13 days (range, 0-533) after diagnosis of SR-LGI GVHD. It was given as >/=3rd line (median 3; range 2-6) in 75% of patients after failure of steroids and additional treatments, that included ruxolitinib (n=12), photopheresis (n=9), sirolimus (n=3), or mesenchymal stromal cells (n=1). Median number of vedolizumab doses given was 2.5 (range 1-5). Median follow-up among survivors was 13.5 months (range, 10.4-33.3).
Adverse events: No PML or infusion reactions occurred. Overall, 44 infection events (22 viral, 18 bacterial, and 4 fungal) were noted in 16 patients. Majority were grade 3 (n=24), followed by grade 1-2 (n=14) and grade 4 (n=6) [Table]. Biochemical liver abnormalities were noted in 5 patients; grade 1 (n=2), grade 2 (n=1), grade 3 (n=2) that peaked between 15-53 days after 1st dose of vedolizumab.
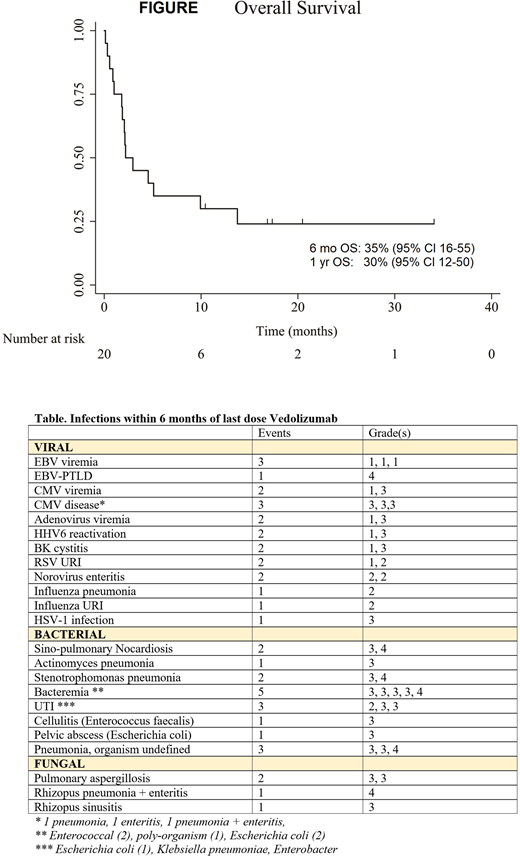
Response: Day 14, 28 and 56 ORR were 45% (9/20; CR 25%), 35% (7/20; CR 20%) and 25% (5/20; CR 20%), respectively. Of 8 patients with no response (NR)/progression at day 14, 3 (37.5%) responded (1CR, 2PR) by day 28 with no additional therapy. Only 1/9 patients with NR/progression at day 28 responded (CR) by day 56 with no additional therapy. Among patients with prior ruxolitinib, day 14, 28 and 56 ORR were 50% (6/12; CR 25%), 50% (6/12; CR 25%) and 25% (3/12; CR 16.7%), respectively. Overall, 15 patients died (14 GVHD, 1 AML relapse). Actuarial OS was 35% (95% CI 16-55) at 6-month and 30% (95% CI 12-50) at 1 year, with a median OS of 2.2 months from start of vedolizumab [Figure].
Conclusion: Vedolizumab was well tolerated, and has potential efficacy even among those with prior ruxolitinib exposure for SR-LGI GVHD. The ORR noted in our series is suboptimal, which is likely a reflection of highly advanced LGI GVHD, and vedolizumab used as >/=3rd line therapy. Earlier use may lead to better outcomes, as seen with previous study [Floisand; BBMT 2019], and as seen with natalizumab for upfront management of LGI GVHD [Kekre Blood 2017].
Mehta:CSL Behring: Research Funding; Kadmon: Research Funding; Incyte: Research Funding. Nieto:Affimed: Consultancy, Other: Grant Support; Astra Zeneca: Other: Grant Support; Novartis: Other: Grant Support; Secura Bio: Other: Grant Support. Oran:Arog Pharmaceuticals: Research Funding; Celgene: Consultancy; ASTEX: Research Funding. Qazilbash:Angiocrine: Research Funding; Bioclinica: Consultancy; Amgen: Research Funding; Bioline: Research Funding; Janssen: Research Funding. Khouri:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding. Rezvani:Pharmacyclics: Other: Educational grant; Affimed: Other: Educational grant; Takeda: Other: Licensing agreement; Virogen: Membership on an entity's Board of Directors or advisory committees; Formula Pharma: Membership on an entity's Board of Directors or advisory committees; Adicet Bio: Membership on an entity's Board of Directors or advisory committees; GemoAb: Membership on an entity's Board of Directors or advisory committees. Popat:Bayer: Research Funding; Novartis: Research Funding. Kebriaei:Amgen: Other: Research Support; Pfizer: Other: Served on advisory board; Ziopharm: Other: Research Support; Novartis: Other: Served on advisory board; Jazz: Consultancy; Kite: Other: Served on advisory board. Shpall:Takeda: Other: Licensing Agreement; Magenta: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Zelluna: Membership on an entity's Board of Directors or advisory committees. Champlin:Genzyme: Speakers Bureau; Omeros: Consultancy; Johnson and Johnson: Consultancy; Cytonus: Consultancy; Takeda: Patents & Royalties; Actinium: Consultancy; DKMS America: Membership on an entity's Board of Directors or advisory committees. Alousi:Incyte: Honoraria, Research Funding; Alexion: Honoraria; Therakos: Research Funding.
Vedolizumab is FDA-approved for treatment of inflammatory bowel disease, but not for GI GVHD. This abstract summarizes our experience with Vedolizumab in the treatment of steroid-refractory GI GVHD.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal