Background: Transplant-eligible MM patients are achieving unprecedented CR rates with frontline therapy. This urges the question about what other tests are informative upon a negative immunofixation (IFx-), as well as if patients with short duration CR continue having dismal survival with modern frontline plus salvage therapies and if so, how to predict risk of unsustained CR.
Aim: To provide an optimal definition of unsustained CR and biomarkers to predict it in transplant-eligible MM patients treated with optimal therapy.
Methods: A total of 262 patients enrolled in the PETHEMA/GEM2012MENOS65 trial and who were in CR after receiving six induction cycles of bortezomib, lenalidomide and dexamethasone (VRD), autologous transplant and two consolidation cycles of VRD, were included in this study. Afterwards, patients were enrolled in the PETHEMA/GEM2014MAIN trial. Median follow-up of the series was 38 months after consolidation (53 months since diagnosis). Serum free light-chains (sFLC) were measured in 252 cases. MRD was assessment with next-generation flow (NGF) in 257 patients (median limit of detection of 2.8x10-6). FISH was performed in CD138-enriched plasma cells (PCs) from 223 patients at diagnosis [high-risk was defined by the presence of t(4;14), t(14;16) and/or del(17p)]. To understand the relationship between duration of CR and outcome, patients were segmented into 6-monht increments (range, 0 - 48 months) in time since response assessment after consolidation and loss of CR.
Results: We first investigated what other tests commonly performed upon the achievement of IFx- were informative, particularly those employed to define stringent CR. The median percentage of PCs by morphology, at the time of IFx-, was 1.7% (range 0-5). Only 4 patients out of 266 (1.5%) with a negative immunofixation had >5% BM PCs and therefore, were not classified as in CR. Almost one-fourth of patients in CR display an abnormal sFLC ratio (56/248, 23%), but their PFS was identical to that of cases with normal sFLC ratio (3 years-PFS rates of 70% vs 72%; P=.6). BM biopsies were not performed in this study to evaluate PC clonality by immunohistochemistry but we noted that in CR patients with persistent MRD, the median percentage of clonal and normal PCs among total PCs identified by NGF was of 3% and 97%, respectively. Thus, the median percentage of normal PCs is 24-fold greater than clonal PCs within the PC compartment, and therefore simple κ/λ ratios measured in ≥100 PCs are unable to detect such low-levels of residual disease. Indeed, persistent MRD was detectable by NGF in 73/252 (29%) CR patients at a median level of 0.03% (range 0.0002% - 0.59%), and resulted in significantly inferior PFS (3-year rates of 49% vs 83% in cases with persistent vs undetectable MRD, P < .00001) and OS (3 years-PFS rates of 84% vs 95%; P=.001).
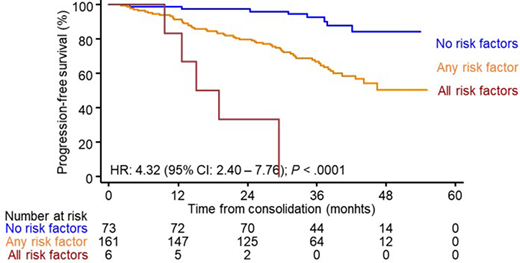
Afterwards, we sought to identify the optimal landmark to define unsustained CR and to identify which biomarkers could identify patients at risk. A duration of CR of <24 months emerged as optimal criteria to identify a numerically relevant subset of patients (39/262, 15%) with dismal OS (median of 48 months vs not reached in patients with duration of CR ≥24 months, P < .00001). We then performed a logistic regression to identify biomarkers with independent value to predict unsustained CR. Elevated LDH levels (HR: 2.9 [1.1 - 8.0], P =.038) and >20% PCs (HR: 2.8 [1.2 - 6.7], P =.020) at diagnosis together with persistent MRD (HR: 4.3 [1.9 - 9.6], P <.001) had independent value to predict unsustained CR in a multivariable analysis. Thus, a scoring system with the three parameters yielded significant stratification of patients in CR into favorable (no risk factors), intermediate (any risk factor) and dismal (all risk factors) PFS (3-year rates of 92%, 67% and 0%, respectively; P <.0001; Figure 1) and OS (3-year rates of 99%, 90% and 83%, respectively; P=.001).
Conclusions: This is the first study evaluating which baseline and response assessments are useful to stratify transplant-eligible patients with unsustained CR in the context of modern therapy; a simple model based on LDH levels and PC counts at diagnosis plus MRD status was identified and can be used broadly. These findings are clinically meaningful because patients with unsustained CR remain a high-risk population despite optimal therapy, and those at risk should be offered alternative treatment strategies before insurmountable disease progression occurs.
Paiva:SkylineDx: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Roche: Research Funding; Adaptive: Honoraria; Amgen: Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Kite: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau. Oriol:Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy; Celgene: Consultancy, Speakers Bureau. Sureda Balari:Roche: Honoraria; Celgene: Consultancy, Honoraria; BMS: Speakers Bureau; Incyte: Consultancy; Janssen: Consultancy, Honoraria; Gilead/Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Merck Sharpe and Dohme: Consultancy, Honoraria, Speakers Bureau; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau. de la Rubia:Janssen: Consultancy, Other: Expert Testimony; Amgen: Consultancy, Other: Expert Testimony; Celgene: Consultancy, Other: Expert Testimony; Ablynx/Sanofi: Consultancy, Other: Expert Testimony. Moraleda:Takeda: Consultancy, Other: Travel Expenses; Sandoz: Consultancy, Other: Travel Expenses; Novartis: Consultancy, Other: Travel Expenses; Gilead: Consultancy, Other: Travel Expenses; Jazz Pharmaceuticals: Consultancy, Research Funding. Mateos:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. San-Miguel:GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal