INTRODUCTION
Development of targeted therapies for chronic lymphocytic leukemia (CLL) including the anti-CD20 monoclonal antibody obinutuzumab (OBIN), Bruton's tyrosine kinase inhibitor ibrutinib (IBR), and Bcl-2 inhibitor venetoclax (VEN) offer highly effective treatment that avoids traditional cytotoxic chemotherapy. Both oral targeted agents (IBR and VEN) have high response rates and long progression-free survival (PFS) as single-agent monotherapy. Fixed duration combinations of IBR and VEN as well as VEN and anti-CD20 monoclonal antibodies have similarly high response rates and frequent achievement of undetectable minimal residual disease (uMRD) status, supporting that prolonged remission after fixed-duration treatment may be possible. Combination therapies have greater toxicities than monotherapy and the benefit of this approach is dependent on the durability of remissions. We conducted a phase 2 study of fixed-duration combination OBIN, IBR, and VEN in separate cohorts of treatment-naïve (TN) and relapsed/refractory (RR) CLL patients and report additional follow up (FU) for disease progression and survival.
METHODS
This study was conducted at the Ohio State University (OSU). Patients with TN or RR (≥1 prior treatment) CLL requiring therapy were eligible. Treatment was administered with OBIN, IBR, and VEN for 14 cycles (C) of 28 days. Drugs were started sequentially with OBIN starting C1, IBR C2, and VEN C3 with standard dose escalation. Final response was determined according to IWCLL 2008 criteria at 2 months after completing C14 (EOT). MRD was measured in the bone marrow and peripheral blood by standard 10-color flow cytometry with a cutoff of <1x10-4 at EOT. The primary endpoint was the rate of complete remission (CR) with uMRD in the blood and the bone marrow at EOT. After EOT, patients were followed for safety and disease assessment every 3 months for the 2 years then every 6 months after that until initiation of alternative therapy, progressive disease (PD), or death. Patients unable to return to OSU for post-treatment visits were assessed locally. The Kaplan-Meier method was used to estimate PFS and overall survival (OS).
RESULTS
The study enrolled 50 patients including 25 TN and 25 RR in separate cohorts (Table 1). Enrollment completed in April of 2017 and data are presented as of July 6th 2020. Forty-three (86%) patients completed planned treatment and underwent EOT assessment. Seven patients did not complete treatment: 3 due to adverse events, 3 patient or physician preference, and 1 concomitant diagnosis of chronic myeloid leukemia. The overall response rate in an intention to treat analysis was 84% (95% CI: 64-95%) in TN and 88% (95% CI: 69-97%) in RR patients. A total of 14 (56%) TN and 11 (44%) RR patients had uMRD in both the blood and bone marrow and 7 (28%) in each group achieved a CR with uMRD.
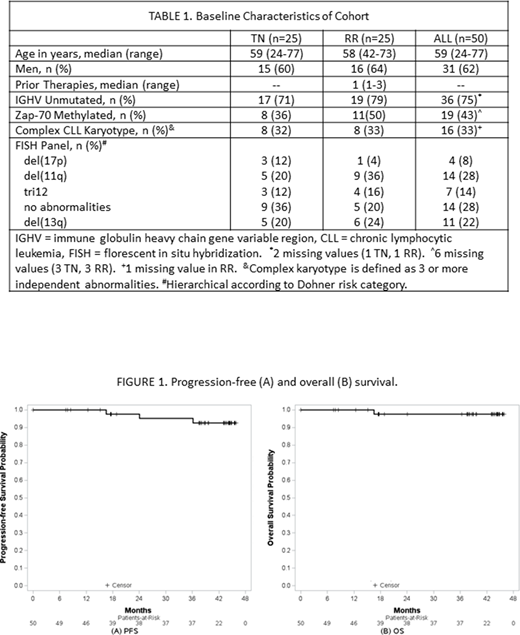
As of the data cut, 70% of patients remain in FU (18 TN, 17 RR). The median FU is 41.4 months (range 7.4-45.7) for TN and 38.8 months (range 0.03-45.9) for RR patients. The medians for PFS and OS were not reached (Figure 1). The 36- month estimated PFS is 95% (95% CI: 72-99%) for TN and 95% (95% CI: 69-99%) for RR patients. The 36-month estimated OS is 95% (95% CI: 72-99) for TN and 100% for RR patients.
One TN patient discontinued treatment after C10 due to treatment-related neutropenic colitis and died 16.7 months after starting treatment. There were no other deaths. No TN and 2 RR patients developed PD at 24 and 37 months, corresponding to 10 and 22 months after EOT. Both had IGHV unmutated CLL with del(13q) and 1 prior treatment with chemoimmunotherapy. The first had a CR with MRD detectable in the bone marrow at EOT and developed immune thrombocytopenia. Bone marrow biopsy revealed 20% CLL. He was treated with IVIG, rituximab, and ibrutinib and remains on ibrutinib with control of his CLL. The second achieved a partial remission with MRD in the bone marrow and developed lymphocytosis. He continues in observation.
CONCLUSIONS
Fixed-duration OBIN, IBR, and VEN in combination results in high rates of overall response and uMRD status. This is the longest FU we are aware of after an IBR/VEN combination regimen and at 36 months remissions remain durable. Further FU is needed to evaluate the long-term durability, MRD kinetics, and factors that predict PFS.
Rogers:AstraZeneca: Consultancy, Other: Travel Funding; Janssen: Research Funding; Pharmacyclics: Consultancy; Genentech: Research Funding; Acerta Pharma: Consultancy; AbbVie: Consultancy, Research Funding. Hoffman:Pharmacyclics: Consultancy; AstraZeneca: Consultancy. Maddocks:Celgene: Consultancy, Honoraria; Karyopharm: Consultancy; Seattle Genetics: Consultancy, Honoraria; BMS: Consultancy, Research Funding; ADC Therapeutics, AstraZeneca: Consultancy; Morphosys: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria. Lozanski:Genentech, Novartis, Beckman Coulter: Research Funding. Woyach:Karyopharm: Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Morphosys: Research Funding; Loxo: Research Funding; Verastem: Research Funding; AbbVie: Research Funding. Byrd:Acerta Pharma: Research Funding; Syndax: Research Funding; Vincera: Research Funding; Novartis: Research Funding; Kartos Therapeutics: Research Funding; Trillium: Research Funding; Leukemia and Lymphoma Society: Other; Janssen: Consultancy; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, BeiGene: Research Funding; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, Novartis, Janssen: Speakers Bureau; Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Gilead, TG Therapeutics: Other.
The three drugs described in this regimen are not approved for use in combination.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal