Background: Venetoclax (ven)+azacitidine (aza) is the standard of care for untreated acute myeloid leukemia (AML) patients (pts) ≥75 years or unfit for intensive induction chemotherapy (IC) due to comorbidities. While the standard of care for younger newly diagnosed pts is IC, long term outcomes for all but favorable risk pts are suboptimal. Specifically, pts with adverse risk disease, per the European Leukemia Network (ELN), are more likely to be refractory to IC and have low rates of long term survival. Ven based regimens have a ~70% response rate in the upfront setting; in contrast to IC, those with adverse risk do not have lower response rates. This study explores the feasibility and outcomes of ven+aza for newly diagnosed younger AML pts who are not necessarily unfit for IC, but carry adverse risk features that make them less likely to respond to this therapy.
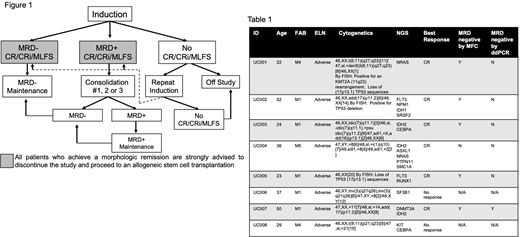
Methods: This is an interim analysis of an ongoing phase 2 study (NCT03573024) of a planned 36 untreated adverse risk AML pts 18-59. Pts have adequate organ function and white blood cell counts <25x109/L (hydrea permitted); "fitness" for IC is not a consideration. In cycle 1, pts receive aza 75mg/m2 on days (d)1-7 and ven, escalated from 100 to 200 to 400 to 600mg on d1-4. Ven continues at 600mg d5-28 and bone marrow biopsies (BMBX) occur on d14 and 28. Pts who achieve morphologic remission without count recovery have up to 14 days off therapy before subsequent cycles. Non responders discontinue or receive up to 1 additional cycle. Post response (defined as a morphologic leukemia free state [MLFS], complete remission [CR] with incomplete count recovery [CRi] or CR), pts who are measurable residual disease (MRD) negative by multiparameter flow cytometry (MFC) and/or droplet digital PCR (ddPCR) may proceed to MRD negative maintenance: ven 400mg d1-28 with aza 75mg/m2 d1-5 of sequential 28-day cycles. Pts who are MRD-positive may receive up to 3 additional cycles of consolidation: ven 600mg/day d1-28 and aza 75mg/m2 d1-7 of a 28-day cycle. Pts who become MRD negative may proceed to MRD negative maintenance. Pts who are MRD positive after induction and consolidation may proceed to MRD positive maintenance: ven 600mg daily d1-28 with aza 75mg/m2 d1-7 of a 28-day cycle. Conversion to MRD negativity results in a transition to MRD-negative maintenance (Fig 1). While these post-remission options are available, pts are encouraged to discontinue the study and proceed to transplant after achieving a morphologic remission. The primary objective is to determine the overall response rate (CR+CRi+MLFS). The endpoint is to show ven+aza is noninferior to historical controls: younger newly diagnosed adverse risk AML pts who receive IC. Frequent analysis of outcomes, using 6 stages, was adopted to ensure the CR rate remains ≥60%.
Results: 8 have enrolled between November 2018 and July 2020; median age is 33 (22-52). All have adverse risk disease (Table 1). There were 179 adverse events (AEs); 35 related. The ≥grade 3 events were: fatigue (N=1), leukopenia (N=2), neutropenia (N=2), anemia (N=2), thrombocytopenia (N=1). There were no deaths in the first 60 days. In cycle 1 pts spent a median 10 days (4-19) inpatient and required a median of 2 (0-10) red blood cell transfusions and 3 (0-15) platelet transfusions. Six of 8 (75%) pts responded; all 6 were CRs. 4/6 responses were cytogenetic remissions, 5/6 were MRD negative by MFC and 1/6 was MRD negative by ddPCR. Two pts relapsed, 35 and 84 days after remission; one was successfully salvaged with IC and proceeded to transplant while the other was refractory to IC and died from AML. Two pts were refractory to ven+aza; both were successfully salvaged with IC and have proceeded to transplant. 7/8 proceeded to transplant; 4 required only ven+aza for this outcome and proceeded to transplant a median of 89 days (56-93) after diagnosis. No pts who have proceeded to transplant have relapsed, a median of 340 days (136-444) after transplant. 7/8 remain alive, 345 days (91-586) after enrolling.
Conclusions: At the stage 1 interim analysis, the CR rate for ven+aza remains >60% in adverse risk younger patients. With small numbers, up-front treatment with ven+aza allows some younger pts with adverse risk disease a non IC opportunity to achieve a response and proceed to transplant; most of those who relapsed or were refractory were salvaged with IC.
Pollyea:Syndax: Consultancy; Syros: Consultancy; Abbvie: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Karyopharm: Consultancy; Novartis: Consultancy; Genentech: Consultancy; Amgen: Consultancy; 47: Consultancy, Research Funding; Janssen: Consultancy; Agios: Consultancy; Glycomimetics: Other; Celgene/BMS: Consultancy; Pfizer: Consultancy; Takeda: Consultancy.
Venetoclax for younger patients fit for induction chemotherapy
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal