Key Points
Patients with cancer treated with immune checkpoint inhibitors are at a substantial risk of developing VTE/ATE.
VTE under immune checkpoint inhibitors strongly impairs clinical outcomes and is difficult to predict.
Abstract
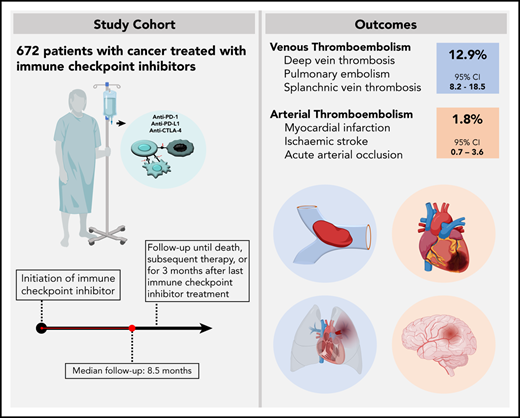
The risk of venous thromboembolism (VTE) and arterial thromboembolism (ATE) associated with immune checkpoint inhibitors is currently unclear. Our aim was to quantify the risk of VTE/ATE in patients with cancer treated with immune checkpoint inhibitors, explore clinical impact, and investigate potential clinical risk factors. Patients treated with immune checkpoint inhibitors at the Medical University of Vienna from 2015 to 2018 were identified using in-house pharmacy records (n = 672; most frequent entities: 30.4% melanoma, 24.1% non-small cell lung cancer; 86% stage IV disease). A retrospective chart review was performed to screen for VTE and/or ATE. Cumulative incidences and between-group differences were estimated in competing-risk analysis. The impact of VTE/ATE on mortality was studied by multistate modelling. Over a median follow-up of 8.5 months, 47 VTEs and 9 ATEs were observed. Cumulative incidences of VTE and ATE were 12.9% (95% confidence interval [CI], 8.2-18.5) and 1.8% (95% CI, 0.7-3.6). Occurrence of VTE was associated with increased mortality (transition hazard ratio, 3.09; 95% CI, 2.07-4.60). History of VTE predicted VTE occurrence (subdistribution hazard ratio [SHR], 3.69; 95% CI, 2.00-6.81), and distant metastasis was nonsignificantly associated with VTE risk (SHR, 1.71; 95% CI, 0.62-4.73). No association of VTE with Eastern Cooperative Oncology Group performance status, Charlson comorbidity index, or Khorana score was observed, and rates of VTE were comparable between tumor types and checkpoint-inhibitory agents. In conclusion, patients with cancer under immune checkpoint inhibitor therapy are at high risk of thromboembolism, especially VTE. Furthermore, VTE occurrence was associated with increased mortality.
Introduction
Patients with cancer are at an increased risk of venous thromboembolism (VTE) and arterial thromboembolism (ATE).1,2 Risk of cancer-associated thrombosis is multifactorial.3 A number of risk factors have been reported, and among them, treatment-related factors such as chemotherapy (eg, platinum based), antiangiogenesis agents, and hormonal therapy have been found to increase the risk of both VTE and ATE in patients with cancer.3-7
With the development and introduction of immune checkpoint inhibitors, new treatment options in medical oncology are now available. Checkpoint inhibitors impair tumoral immune-escape mechanisms by targeting programmed cell death protein 1 (PD-1) or its ligand (PD-L1) or cytotoxic T-lymphocyte–associated protein 4 (CTLA-4). The induction of a strong systemic antitumor immune response has led to substantial improvement of prognosis in patients with melanoma, non-small cell lung cancer, renal cell carcinoma, head and neck squamous cell carcinoma, and other cancers.8-12 However, this is accompanied by various off-target manifestations of autoimmunity induced by immune checkpoint inhibitors.13 The impact of immune checkpoint inhibition induced systemic inflammation on the hemostatic system has not been properly investigated to date. Furthermore, results from randomized controlled trials evaluating the efficacy of immune checkpoint inhibitors for treatment of various cancers did not report rates of VTE and ATE.14 In recently published, small and retrospective cohort studies, rates of VTE in patients receiving immune checkpoint inhibitors were between 6% and 18%, and several case reports described dramatic and fatal thromboembolic events during immune checkpoint inhibitor therapy.15-19 However, the risk of VTE and ATE associated with treatment using these new anticancer agents and its impact on patient prognosis remain unclear.
Therefore, the aim of this study was to investigate the frequency, potential risk factors, and clinical consequences of VTE and ATE in a large and unselected single-institutional cohort of patients treated with immune checkpoint inhibitors.
Methods
Study design and cohort derivation
We conducted a single-center retrospective cohort study at the Vienna General Hospital of the Medical University of Vienna, Vienna, Austria. The detailed protocol of all study-related procedures and analyses has been approved by the institutional ethics committee (number 2213/2019; ethik-kom@meduniwien.ac.at). The study was conducted in accordance with the Declaration of Helsinki.
The study cohort comprises adult patients (≥18 years of age) with histologically confirmed cancer who were treated with ≥1 dose of an approved immune checkpoint inhibitor (nivolumab, pembrolizumab, ipilimumab, atezolizumab, or avelumab) between January 2015 and November 2018. Patients were identified using the in-house pharmacy prescription program. Patients enrolled in blinded randomized controlled trials were excluded due to uncertainty of therapy assignment. Patients receiving immune checkpoint inhibitors in open-label or single-arm interventional trials were eligible for inclusion. Patients with a prior history of thrombotic events or continuous anticoagulation were not excluded in order to represent a “real-life” setting for risk evaluation of thrombotic events under immune checkpoint inhibitor treatment.
Study procedures and outcomes
Data on baseline demographics, comorbidities, tumor specifics, prior antineoplastic therapy, and outcome were collected by electronic chart review. The primary outcomes of the study were cumulative incidence rates of VTE and ATE. Occurrence of VTE was defined in accordance to the Vienna Cancer and Thrombosis Study and comprises acute symptomatic or incidental deep vein thrombosis (DVT), pulmonary embolism (PE), splanchnic vein thrombosis, and fatal PE.20 ATE was defined as acute coronary syndrome, acute peripheral artery occlusion, and ischemic stroke.2 The observation period for the occurrence of VTE and/or ATE started at the first day of immune checkpoint inhibitor treatment and was terminated either with the initiation of any subsequent antineoplastic medical therapy such as chemotherapy or 3 months after the last cycle of therapy. This timeframe is in accordance with clinical trials testing immune checkpoint inhibitors with a median time of reporting adverse events after the last therapy cycle of 90 days.21 Details on our predefined primary outcome definitions are provided in the supplemental Appendix (supplemental Table 1, available on the Blood Web site).
Diagnosis of each thrombotic event had to be verified by objective diagnostic imaging tests and was confirmed by an independent adjudication committee. Objective measures for diagnosis of VTE and ATE are summarized in supplemental Table 1.
Secondary outcomes comprise the association of VTE/ATE with overall survival (OS), progression-free survival (PFS), and radiological disease control rate (DCR).22 Additional secondary outcomes include efficacy (rate of recurrent VTE) and safety (rate of major bleeding and clinically relevant non-major bleeding)23,24 of therapeutic anticoagulation for VTE and the impact of VTE/ATE on treatment discontinuation and/or delay. Detailed definitions of secondary outcomes are provided in supplemental Table 2.
Statistical analysis
Standard summary statistics were applied for reporting patient baseline variables (absolute frequencies/percentages; median/interquartile range [IQR]). Median follow-up was calculated by the reverse Kaplan-Meier-method. Thrombotic end points were studied within a competing-risk framework, treating all-cause mortality as a competing outcome event. Cumulative incidences were calculated by the competing risk estimator and corresponding standard errors according to Marubini and Valsecci, utilizing Gray’s test for subgroup comparisons.25,26 Uni- and multivariable modeling of time to event was conducted within a proportional subhazard regression model according to Fine and Gray.27 Survival times (OS and PFS) were estimated by the method of Kaplan-Meier. The association of VTE/ATE with OS and PFS was studied within a multistate model, treating thrombotic events as time-dependent covariables and reporting corresponding transition hazard ratios (THR).28 A landmark analysis was conducted comparing OS of patients experiencing VTE in the first 3 months of observations to those who did not. The Mantel-Byar test was used for comparisons of post-event survival times. The association of VTE/ATE with DCR was evaluated by binary logistic regression. All statistical analyses were performed with the commercially available package STATA 15.0 (Stata, Houston, TX).
Results
Description and characteristics of the study population
Between 2015 and 2018, 672 patients received treatment with an immune checkpoint inhibitor at our institution either in routine care (n = 580) or in open-label or single-arm interventional trials (n = 92). Median age at therapy initiation was 64 years (IQR, 54-72 years), and 38.7% were female. Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (92.4%) and the median Charlson comorbidity index was 8 (IQR, 7-9), with 6 points allocated to metastatic cancer. Eighty-five patients (12.6%) had a history of VTE prior to the initiation of immune checkpoint inhibitor therapy. Of those, 65 patients (8.0% of the total cohort) experienced prior VTE associated with the current cancer diagnosis. History of ATE was positive in 62 patients (9.2%) and was associated with the current cancer diagnosis in 15 patients (2.2% of the total cohort). At the initiation of immune checkpoint inhibitor therapy, 111 patients (16.5%) underwent continuous anticoagulation and 133 patients (19.8%) received antiplatelet therapy, with details on indication and agents provided in supplemental Table 3.
The most frequent cancer types of patients included in the study were malignant melanoma (n = 204, 30.4%), non-small cell lung cancer (n = 162, 24.1%), renal cell carcinoma (n = 74, 11.0%), head and neck squamous cell carcinoma (n = 70, 10.4%), and urothelial cancer (n = 33, 4.9%). Most patients had distant metastasis at therapy initiation (n = 566, 85.8%). The most frequently used immune checkpoint inhibitor was nivolumab (n = 282, 42.0%), followed by pembrolizumab (n = 269, 40.0%), ipilimumab (n = 45, 6.7%), ipilimumab in combination with nivolumab (n = 40, 6.0%), atezolizumab (n = 30, 4.5%), and avelumab (n = 6, 0.9%). The median number of therapy cycles administered was 7 (IQR, 4-18; range, 1-128). Most patients underwent chemotherapy prior to the initiation of immune checkpoint inhibitors (n = 377, 56.1%), and 320 patients (47.6%) were treated with radiation therapy previously. Eighty-four patients (12.5%) underwent a second line and 19 patients (2.8%) a third line of immune checkpoint inhibitor therapy. Following immune checkpoint inhibitors, 210 patients (31.3%) underwent subsequent antineoplastic therapy. Details on cancer diagnosis, patient characteristics, and treatment are provided in Table 1.
Baseline characteristics of the study cohort (n = 672)
| Variable . | n (% missing) . | Median [IQR] or count (%) . |
|---|---|---|
| Demographics and clinical characteristics | ||
| Age (y) | 672 (0) | 64 [54-72] |
| Female | 672 (0) | 260 (38.7) |
| BMI (kg/m2) | 545 (18.9) | 24.4 [21.5-28.0] |
| ECOG | 553 (17.7) | 0 [0-1] |
| 0-1 | — | 511 (92.4) |
| ≥2 | — | 42 (7.6) |
| Charlson comorbidity index | 672 (0) | 8 [7-9] |
| History of VTE* | 672 (0) | 85 (12.6) |
| History of VTE during current cancer disease | 672 (0) | 65 (8.0) |
| History of ATE* | 672 (0) | 62 (9.2) |
| History of ATE during current cancer disease | 672 (0) | 15 (2.2) |
| Continuous anticoagulation | 672 (0) | 111 (16.5) |
| Continuous antiplatelet therapy | 672 (0) | 133 (19.8) |
| Tumor specifics at inclusion | ||
| Tumor type | 672 (0) | — |
| Melanoma | — | 204 (30.4) |
| Non-small cell lung cancer | — | 162 (24.1) |
| Renal cell carcinoma | — | 74 (11.0) |
| Head and neck squamous cell carcinoma | — | 70 (10.4) |
| Urothelial | — | 33 (4.9) |
| Lymphoma/myeloma | — | 28 (4.2) |
| Hepatocellular cancer | — | 20 (3.0) |
| Gynecological | — | 18 (2.7) |
| Sarcoma | — | 17 (2.5) |
| Colorectal cancer | — | 11 (1.6) |
| Other† | — | 35 (5.2) |
| Stage | 660 (1.8) | — |
| I | — | 3 (0.5) |
| II | — | 13 (2.0) |
| III | — | 78 (11.8) |
| IV | — | 566 (85.8) |
| PD-L1 (TPS) | 178 (73.5) | 10 [0-60] |
| PD-L1 negative | — | 58 (32.6) |
| Therapeutic management | ||
| Immune checkpoint inhibitor agent | 672 (0) | — |
| Nivolumab | — | 282 (42.0) |
| Pembrolizumab | — | 269 (40.0) |
| Ipilimumab | — | 45 (6.7) |
| Atezolizumab | — | 30 (4.5) |
| Avelumab | — | 6 (0.9) |
| Ipilimumab + nivolumab | — | 40 (6.0) |
| Therapy cycles | — | 7 [4-18], range: 1-128 |
| Treatment intent | — | — |
| (Pseudo-)neoadjuvant | — | 1 (0.1) |
| (Pseudo-)adjuvant | — | 19 (2.8) |
| Palliative | — | 651 (96.9) |
| Line of anticancer therapy | — | 2 [1-2], range: 1-7 |
| Prior chemotherapy | — | 377 (56.1) |
| Prior radiotherapy | — | 320 (47.6) |
| Prior removal of primary tumor | — | 357 (53.1) |
| Concomitant therapy during immune checkpoint inhibitor therapy | — | — |
| Chemotherapy | — | 43 (6.4) |
| Targeted therapy | — | 69 (10.3) |
| Radiotherapy | — | 107 (15.9) |
| Surgery | — | 43 (6.4) |
| Medical anticancer therapy after immune checkpoint inhibitor therapy | — | 210 (31.3) |
| Variable . | n (% missing) . | Median [IQR] or count (%) . |
|---|---|---|
| Demographics and clinical characteristics | ||
| Age (y) | 672 (0) | 64 [54-72] |
| Female | 672 (0) | 260 (38.7) |
| BMI (kg/m2) | 545 (18.9) | 24.4 [21.5-28.0] |
| ECOG | 553 (17.7) | 0 [0-1] |
| 0-1 | — | 511 (92.4) |
| ≥2 | — | 42 (7.6) |
| Charlson comorbidity index | 672 (0) | 8 [7-9] |
| History of VTE* | 672 (0) | 85 (12.6) |
| History of VTE during current cancer disease | 672 (0) | 65 (8.0) |
| History of ATE* | 672 (0) | 62 (9.2) |
| History of ATE during current cancer disease | 672 (0) | 15 (2.2) |
| Continuous anticoagulation | 672 (0) | 111 (16.5) |
| Continuous antiplatelet therapy | 672 (0) | 133 (19.8) |
| Tumor specifics at inclusion | ||
| Tumor type | 672 (0) | — |
| Melanoma | — | 204 (30.4) |
| Non-small cell lung cancer | — | 162 (24.1) |
| Renal cell carcinoma | — | 74 (11.0) |
| Head and neck squamous cell carcinoma | — | 70 (10.4) |
| Urothelial | — | 33 (4.9) |
| Lymphoma/myeloma | — | 28 (4.2) |
| Hepatocellular cancer | — | 20 (3.0) |
| Gynecological | — | 18 (2.7) |
| Sarcoma | — | 17 (2.5) |
| Colorectal cancer | — | 11 (1.6) |
| Other† | — | 35 (5.2) |
| Stage | 660 (1.8) | — |
| I | — | 3 (0.5) |
| II | — | 13 (2.0) |
| III | — | 78 (11.8) |
| IV | — | 566 (85.8) |
| PD-L1 (TPS) | 178 (73.5) | 10 [0-60] |
| PD-L1 negative | — | 58 (32.6) |
| Therapeutic management | ||
| Immune checkpoint inhibitor agent | 672 (0) | — |
| Nivolumab | — | 282 (42.0) |
| Pembrolizumab | — | 269 (40.0) |
| Ipilimumab | — | 45 (6.7) |
| Atezolizumab | — | 30 (4.5) |
| Avelumab | — | 6 (0.9) |
| Ipilimumab + nivolumab | — | 40 (6.0) |
| Therapy cycles | — | 7 [4-18], range: 1-128 |
| Treatment intent | — | — |
| (Pseudo-)neoadjuvant | — | 1 (0.1) |
| (Pseudo-)adjuvant | — | 19 (2.8) |
| Palliative | — | 651 (96.9) |
| Line of anticancer therapy | — | 2 [1-2], range: 1-7 |
| Prior chemotherapy | — | 377 (56.1) |
| Prior radiotherapy | — | 320 (47.6) |
| Prior removal of primary tumor | — | 357 (53.1) |
| Concomitant therapy during immune checkpoint inhibitor therapy | — | — |
| Chemotherapy | — | 43 (6.4) |
| Targeted therapy | — | 69 (10.3) |
| Radiotherapy | — | 107 (15.9) |
| Surgery | — | 43 (6.4) |
| Medical anticancer therapy after immune checkpoint inhibitor therapy | — | 210 (31.3) |
BMI, body mass index; MMR-d, mismatch repair deficiency; MSI, microsatellite instability; TPS, tumor proportion score.
History of VTE/ATE comprises all reported thrombotic events reported prior to the initiation of immune checkpoint inhibitors.
Includes prostate cancer (n = 8), Merkel cell carcinoma (n = 5), malignant pleural mesothelioma (n = 4), gastroesophageal cancer (n = 4), breast cancer (n = 4), small cell lung cancer (n = 3), cancer of unknown primary (n = 2), glioblastoma (n = 2), penile carcinoma (n = 1), glioma (n = 1), and thyroid carcinoma (n = 1).
Frequency of VTE and ATE
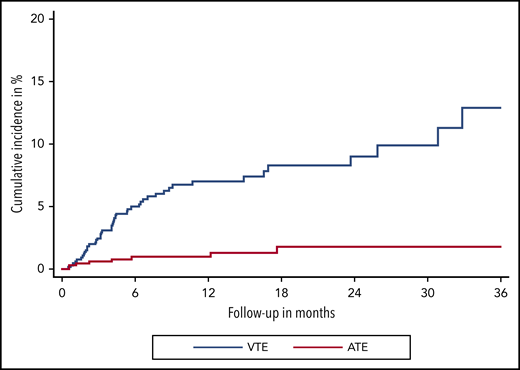
Over a median follow-up of 8.5 months (IQR, 7.6-9.6), 47 VTE events were observed. In competing-risk analysis, the cumulative incidence of VTE under immune checkpoint inhibitor therapy was 12.9% (95% confidence interval [CI], 8.2-18.5) (Figure 1). Median time to VTE was 4.2 months (95% CI, 3.1-5.4). Corresponding 3-, 6-, 12-, and 24-month cumulative incidences of VTE were 2.4% (95% CI, 1.4-3.8), 5.0% (95% CI, 3.4-6.9), 7.0% (95% CI, 5.1-9.3), and 9.0% (95%CI. 6.4-12.1), respectively. The most frequent types of VTE were PE in 18 patients and DVT in 17 patients. DVT in combination with PE occurred in 3 additional patients, catheter-related thrombosis in 4, and visceral vein thrombosis in 5 patients. One patient experienced fatal PE, and 1 patient died under the clinical suspicion of PE (Table 2).
Cumulative incidence functions of VTE and ATE. Cumulative incidence functions are obtained within a competing risk framework, considering all-cause mortality as the competing event of interest.
Cumulative incidence functions of VTE and ATE. Cumulative incidence functions are obtained within a competing risk framework, considering all-cause mortality as the competing event of interest.
Clinical characteristics of VTE and ATE
| . | n (%) . |
|---|---|
| VTE (47 events) | |
| Type | |
| DVT | 17 (36.2) |
| PE | 18 (38.3) |
| DVT + PE | 3 (6.4) |
| Splanchnic vein thrombosis | 5 (10.6) |
| Catheter-related thrombosis | 4 (8.5) |
| Symptoms | |
| Symptomatic | 19 (40.4) |
| Incidental | 22 (46.8) |
| No information regarding symptoms available | 4 (8.5) |
| Fatal* | 2 (4.3) |
| ATE (9 events) | |
| Type | |
| STEMI | 2 (22.2) |
| NSTEMI | 2 (22.2) |
| Ischemic stroke | 3 (33.3) |
| Acute vascular occlusion | 2 (22.2) |
| Symptoms | |
| Symptomatic | 7 (77.8) |
| No information regarding symptoms available | 1 (11.1) |
| Fatal | 1 (11.1) |
| . | n (%) . |
|---|---|
| VTE (47 events) | |
| Type | |
| DVT | 17 (36.2) |
| PE | 18 (38.3) |
| DVT + PE | 3 (6.4) |
| Splanchnic vein thrombosis | 5 (10.6) |
| Catheter-related thrombosis | 4 (8.5) |
| Symptoms | |
| Symptomatic | 19 (40.4) |
| Incidental | 22 (46.8) |
| No information regarding symptoms available | 4 (8.5) |
| Fatal* | 2 (4.3) |
| ATE (9 events) | |
| Type | |
| STEMI | 2 (22.2) |
| NSTEMI | 2 (22.2) |
| Ischemic stroke | 3 (33.3) |
| Acute vascular occlusion | 2 (22.2) |
| Symptoms | |
| Symptomatic | 7 (77.8) |
| No information regarding symptoms available | 1 (11.1) |
| Fatal | 1 (11.1) |
AP, angina pectoris; CRT, catheter-related thrombosis; CVA, cerebral vascular attack (ischemic stroke); NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Includes 1 patient who died under the clinical suspicion of PE, and PE could not be ruled out as a cause of death.
During our observation period, 9 ATEs were observed (Figure 1). The cumulative incidence of ATE at 3, 6, 12, and 24 months was 0.6% (95% CI, 0.2-1.5), 1.0% (95% CI, 0.4-2.0), 1.3% (95% CI, 0.5-2.6), and 1.8% (95% CI, 0.7-3.6), respectively. ATE events, which occurred during immune checkpoint inhibitor therapy, were acute coronary syndrome in 4 patients, ischemic stroke in 3 patients, and acute vascular occlusion in 2 patients. One patient had a confirmed fatal ATE (ischemic stroke) (Table 2).
Association of VTE and ATE with survival and therapy response
Over a median follow-up for survival of 23.1 months, 294 deaths were recorded. Median OS of patients was 25.4 months (95% CI, 21.0-38.6), and median PFS was 6.2 months (95% CI, 5.1-7.4).
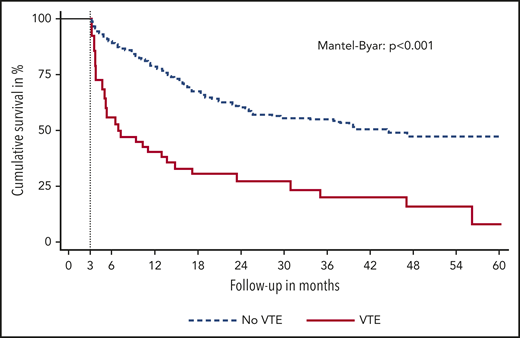
The occurrence of VTE was associated with shorter OS (THR for death, 3.09; 95% CI, 2.07-4.60). Median OS after the occurrence of VTE was 11.6 months compared with 25.5 months in those without VTE (Mantel-Byar P < .001). The diagnosis of VTE was further associated with shorter PFS (THR for progression of disease, 3.63; 95% CI, 2.47-5.36). Median PFS after VTE was 1.7 months compared with 6.7 months in those without VTE (Mantel-Byar P < .001). Figure 2 displays a landmark analysis of OS stratified by the occurrence of VTE within the first 3 months of immune checkpoint inhibitor therapy. No significant differences in best radiological therapy response were observed for patients with VTE vs those without (odds ratio for DCR, 0.84; 95% CI, 0.43-1.63).
Landmark analysis of OS. Patients are stratified by the occurrence of VTE in the first 3 months of immune checkpoint inhibitor therapy.
Landmark analysis of OS. Patients are stratified by the occurrence of VTE in the first 3 months of immune checkpoint inhibitor therapy.
Occurrence of ATE was not associated with risk of mortality (THR, 0.79; 95% CI, 0.25-2.48) or early progression of disease (THR, 0.64; 95% CI, 0.16-2.57), and no association with best radiological treatment response was observed (odds ratio for DCR, 1.17; 95% CI, 0.31-4.39).
Subgroup analyses
Cumulative incidences of thrombotic events and corresponding subdistribution hazard ratios (SHRs) were estimated for subgroups of patients according to cancer entity and type of immune checkpoint inhibitor. The cumulative incidence of VTE was 12.9% (95% CI, 6.6-21.4) in patients with melanoma (n = 204; 16 VTE), 11.7% (95% CI, 5.4-20.7) in patients with non-small cell lung cancer (n = 162; 12 VTE), 4.9% (95% CI, 1.2-12.2) in patients with renal cell carcinoma (n = 74; 3 VTE), 8.7% (95% CI, 2.7-19.2) in patients with head and neck squamous cell carcinoma (n = 70, 4 VTE), 15.7% (95% CI, 3.9-34.8) in patients with hepatocellular cancer (n = 20, 3 VTE), and 26.9% (95% CI, 7.7-51.1) in patients with gynecological cancer (n = 18; 4 VTE).
One VTE event each occurred in patients with urothelial cancer (n = 33), lymphoma (n = 28), sarcoma (n = 17), Merkel cell carcinoma (n = 5), and glioblastoma (n = 2).
No differences in VTE risk between subgroups of tumor types and the remainder of the cohort as reference cohort were observed for patients with melanoma (SHR, 0.99; 95% CI, 0.55-1.80), non-small cell lung cancer (SHR, 1.32; 95% CI, 0.69-2.56), renal cell carcinoma (SHR, 0.55; 95% CI, 0.17-1.77), head and neck squamous cell carcinoma (SHR 0.90; 95% CI, 0.32-2.49), and hepatocellular cancer (SHR, 2.28; 95% CI, 0.68-7.68). Patients with gynecological cancer were at higher risk of VTE compared with the remainder of patients (SHR, 4.08; 95% CI, 1.43-11.63).
Risk of VTE was comparable between different immune checkpoint inhibitory agents, with a cumulative incidence of 9.8% (95% CI, 5.9-14.8) for nivolumab (n = 282; 20 VTE), 13.9% (95% CI, 5.4-26.4) for pembrolizumab (n = 269; 17 VTE), 13.6% (95% CI, 5.8-24.7) for ipilimumab monotherapy (n = 45; 7 VTE), and 19.9% (95% CI, 3.0-47.4) for atezolizumab (n = 30, 3 VTE), with corresponding SHRs for patients treated with the agent against the remainder of patients of 1.08 (95% CI, 0.61-1.92), 0.89 (95% CI, 0.49-1.61), 1.02 (95% CI, 0.50-2.09), and 1.36 (95% CI, 0.43-4.26), respectively. No VTE events were observed in patients treated with ipilimumab-nivolumab combination (n = 40) or avelumab (n = 6).
Occurrence of ATE was observed in 2 patients with melanoma, 2 patients with non-small cell lung cancer, 2 patient with renal cell carcinoma, and 1 patient with gynecological cancer, lymphoma, and prostate cancer, respectively. ATE occurred in 4 patients treated with nivolumab and pembrolizumab and 1 patient treated with atezolizumab. Detailed results of these subgroup analyses are displayed in Table 3.
Subgroup analyses
| Subgroup . | VTE (47 total) . | Cumulative VTE incidence in % (95% CI) . | SHR (95% CI) . | ATE (9 total) . | Cumulative ATE incidence in % (95% CI) . | SHR (95% CI) . |
|---|---|---|---|---|---|---|
| Tumor type | ||||||
| Melanoma (n = 204) | 16 | 12.9 (6.6-21.4) | 0.99 (0.55-1.80) | 2 | 1.6 (0.3-5.7) | 0.52 (0.11-2.54) |
| Non-small cell lung cancer (n = 162) | 12 | 11.7 (5.4-20.7) | 1.32 (0.69-2.56) | 2 | 1.4 (0.3-4.4) | 1.14 (0.24-5.34) |
| Renal cell carcinoma (n = 74) | 3 | 4.9 (1.2-12.2) | 0.55 (0.17-1.77) | 2 | 1.7 (0.1-7.8) | 1.20 (0.15-9.52) |
| Head and neck squamous cell carcinoma (n = 70) | 4 | 8.7 (2.7-19.2) | 0.90 (0.32-2.49) | 0 | — | — |
| Urothelial (n = 33) | 1 | — | — | 0 | — | — |
| Lymphoma (n = 28) | 1 | — | — | 1 | — | — |
| Hepatocellular cancer (n = 20) | 3 | 15.7 (3.9-34.8) | 2.28 (0.68-7.68) | 0 | — | — |
| Gynecological cancer (n = 18) | 4 | 26.9 (7.7-51.1) | 4.08 (1.43-11.63) | 1 | — | — |
| Sarcoma (n = 17) | 1 | — | — | 0 | — | — |
| Gastrointestinal cancer (upper and lower, n = 15) | 0 | — | — | 0 | — | — |
| Prostate cancer | 0 | — | — | 1 | — | |
| Merkel cell carcinoma (n = 5) | 1 | — | — | 0 | — | — |
| Glioblastoma (n = 2) | 1 | — | — | 0 | — | |
| Immune checkpoint inhibitor agent | ||||||
| Nivolumab (n = 282) | 20 | 9.8 (5.9-14.8) | 1.08 (0.61-1.92) | 4 | 2.5 (0.6-7.0) | 0.85 (0.20-3.60) |
| Pembrolizumab (n = 269) | 17 | 13.9 (5.4-26.4) | 0.89 (0.49-1.61) | 4 | 1.5 (0.5-3.7) | 1.33 (0.37-4.76) |
| Ipilimumab (n = 45) | 7 | 13.6 (5.8-24.7) | 1.02 (0.50-2.09) | 0 | — | — |
| Ipilimumab + nivolumab (n = 40) | 0 | — | — | 0 | — | — |
| Atezolizumab (n = 30) | 3 | 19.9 (3.0-47.4) | 1.36 (0.43-4.26) | 1 | — | — |
| Avelumab (n = 6) | 0 | — | — | 0 | — | — |
| Subgroup . | VTE (47 total) . | Cumulative VTE incidence in % (95% CI) . | SHR (95% CI) . | ATE (9 total) . | Cumulative ATE incidence in % (95% CI) . | SHR (95% CI) . |
|---|---|---|---|---|---|---|
| Tumor type | ||||||
| Melanoma (n = 204) | 16 | 12.9 (6.6-21.4) | 0.99 (0.55-1.80) | 2 | 1.6 (0.3-5.7) | 0.52 (0.11-2.54) |
| Non-small cell lung cancer (n = 162) | 12 | 11.7 (5.4-20.7) | 1.32 (0.69-2.56) | 2 | 1.4 (0.3-4.4) | 1.14 (0.24-5.34) |
| Renal cell carcinoma (n = 74) | 3 | 4.9 (1.2-12.2) | 0.55 (0.17-1.77) | 2 | 1.7 (0.1-7.8) | 1.20 (0.15-9.52) |
| Head and neck squamous cell carcinoma (n = 70) | 4 | 8.7 (2.7-19.2) | 0.90 (0.32-2.49) | 0 | — | — |
| Urothelial (n = 33) | 1 | — | — | 0 | — | — |
| Lymphoma (n = 28) | 1 | — | — | 1 | — | — |
| Hepatocellular cancer (n = 20) | 3 | 15.7 (3.9-34.8) | 2.28 (0.68-7.68) | 0 | — | — |
| Gynecological cancer (n = 18) | 4 | 26.9 (7.7-51.1) | 4.08 (1.43-11.63) | 1 | — | — |
| Sarcoma (n = 17) | 1 | — | — | 0 | — | — |
| Gastrointestinal cancer (upper and lower, n = 15) | 0 | — | — | 0 | — | — |
| Prostate cancer | 0 | — | — | 1 | — | |
| Merkel cell carcinoma (n = 5) | 1 | — | — | 0 | — | — |
| Glioblastoma (n = 2) | 1 | — | — | 0 | — | |
| Immune checkpoint inhibitor agent | ||||||
| Nivolumab (n = 282) | 20 | 9.8 (5.9-14.8) | 1.08 (0.61-1.92) | 4 | 2.5 (0.6-7.0) | 0.85 (0.20-3.60) |
| Pembrolizumab (n = 269) | 17 | 13.9 (5.4-26.4) | 0.89 (0.49-1.61) | 4 | 1.5 (0.5-3.7) | 1.33 (0.37-4.76) |
| Ipilimumab (n = 45) | 7 | 13.6 (5.8-24.7) | 1.02 (0.50-2.09) | 0 | — | — |
| Ipilimumab + nivolumab (n = 40) | 0 | — | — | 0 | — | — |
| Atezolizumab (n = 30) | 3 | 19.9 (3.0-47.4) | 1.36 (0.43-4.26) | 1 | — | — |
| Avelumab (n = 6) | 0 | — | — | 0 | — | — |
Cumulative incidences were only estimated if ≥2 events occurred in a specific subgroup. Otherwise, only the crude number of events is reported. Only entities with a minimum number of 10 patients or occurrence of a thrombotic event are listed. Not listed are small cell lung cancer (n = 3), cancer of unknown primary (n = 2), pleural mesothelioma (n = 4), breast cancer (n = 3), penile carcinoma (n = 1), glioma (n = 1), and thyroid cancer (n = 1).
Risk factors for VTE under immune checkpoint inhibitors
The association of clinical risk factors for VTE with immune checkpoint inhibitor therapy was explored. A positive history of VTE was associated with an elevated risk of VTE (SHR, 3.69; 95% CI, 2.00-6.81), with a prior event in 10 of 47 VTE patients (21%; 8 cancer associated and 2 unrelated to cancer). Of those, 5 VTEs occurred despite continuous anticoagulation at the time of VTE recurrence.
A nonsignificant difference in VTE risk was observed between patients with metastatic disease (ie, stage IV; n = 567, 42 VTEs) and stages I to III (n = 93, 4 VTEs) (SHR, 1.71; 95% CI, 0.62-4.73; cumulative incidence estimates: stage IV, 13.6% [95% CI, 8.6-19.7], stage I to III, 5.4% [95% CI, 1.7-12.2]; Gray’s test: P = .274). No association between ECOG performance status (SHR for ECOG ≥1 vs 0, 0.92; 95% CI, 0.43-1.95), Charlson comorbidity index (SHR for 1-point increase, 1.05; 95% CI, 0.94-1.17), sex (SHR for male vs female, 1.48; 95% CI, 0.79-2.76), age (SHR per 10-year increase, 1.04; 95% CI, 0.87-1.27), BMI (SHR per unit increase, 1.04; 95% CI, 0.99-1.09), and histological grade (SHR, 0.55; 95% CI, 0.27-1.15) with VTE was found. Further, the Khorana score did not predict risk of VTE (SHR per point increase, 0.93; 95% CI, 0.60-1.46; SHR for score of ≥2 compared with 0-1 points, 0.69; 95% CI, 0.32-1.51). Also, the expression levels of PD-L1 on tumors cells were not significantly associated with risk of VTE (SHR per 10% increase in TPS, 1.10; 95% CI, 0.97-1.26). No association with VTE risk was observed for patients undergoing continuous anticoagulation (SHR, 1.11; 95% CI, 0.52-2.36) or anti-platelet therapy (SHR, 1.17; 95% CI, 0.58-2.35) at baseline. Table 4 summarizes the results of these risk factor explorations.
Exploration of risk factors for VTE
| Risk factor . | Association with risk of VTE, SHR (95% CI) . |
|---|---|
| History of VTE | 3.69 (2.00-6.81), P < .001 |
| Stage of disease (stage IV vs I-III) | 1.71 (0.62-4.73), P = .303 |
| ECOG performance status (≥1 vs 0) | 0.92 (0.43-1.95), P = .828 |
| Charlson comorbidity index (per point increase) | 1.05 (0.94-1.17), P = .366 |
| Grade | 0.55 (0.27-1.15), P = .112 |
| Sex (male vs female) | 1.48 (0.79-2.76), P = .221 |
| Age (per 10-y increase) | 1.04 (0.87-1.23), P = .684 |
| BMI (per point increase) | 1.04 (0.99-1.09), P = .066 |
| Occurrence of immune-related adverse event | 0.78 (0.41-1.49), P = .457 |
| Khorana score (per 1-point increase) | 0.93 (0.60-1.46), P = .761 |
| Khorana score (≥2 vs 0-1) | 0.69 (0.32-1.51), P = .353 |
| PD-L1 TPS (per 10% increase) | 1.10 (0.97-1.26), P = .147 |
| Anticoagulation at baseline | 1.11 (0.52-2.36) P = .793 |
| Antiplatelet therapy at baseline | 1.17 (0.58-2.35), P = .654 |
| Risk factor . | Association with risk of VTE, SHR (95% CI) . |
|---|---|
| History of VTE | 3.69 (2.00-6.81), P < .001 |
| Stage of disease (stage IV vs I-III) | 1.71 (0.62-4.73), P = .303 |
| ECOG performance status (≥1 vs 0) | 0.92 (0.43-1.95), P = .828 |
| Charlson comorbidity index (per point increase) | 1.05 (0.94-1.17), P = .366 |
| Grade | 0.55 (0.27-1.15), P = .112 |
| Sex (male vs female) | 1.48 (0.79-2.76), P = .221 |
| Age (per 10-y increase) | 1.04 (0.87-1.23), P = .684 |
| BMI (per point increase) | 1.04 (0.99-1.09), P = .066 |
| Occurrence of immune-related adverse event | 0.78 (0.41-1.49), P = .457 |
| Khorana score (per 1-point increase) | 0.93 (0.60-1.46), P = .761 |
| Khorana score (≥2 vs 0-1) | 0.69 (0.32-1.51), P = .353 |
| PD-L1 TPS (per 10% increase) | 1.10 (0.97-1.26), P = .147 |
| Anticoagulation at baseline | 1.11 (0.52-2.36) P = .793 |
| Antiplatelet therapy at baseline | 1.17 (0.58-2.35), P = .654 |
Clinical outcomes and anticoagulation
Of 47 patients developing VTE, the most frequently used anticoagulation therapy was low-molecular-weight heparin (n = 22, 47%), followed by direct oral anticoagulants (n = 13, 28%) and low-molecular-weight heparin transitioning to direct oral anticoagulants (n = 8; 17%). Unfractionated heparin was used as initial treatment in 1 patient with consequently suspected fatal PE, and no anticoagulation was applied in 3 patients due to fatal PE (n = 1), bleeding risk (n = 1), or death unrelated to VTE shortly after the index event (n = 1).
After the index VTE, 4 out of 47 patients (8.5%) experienced VTE recurrence, and 6 patients (12.8%) experienced hemorrhage during anticoagulation (major bleeding, n = 2 [4.3%]; clinically relevant non-major bleeding, n = 4 [8.5%]). Details on anticoagulation, VTE recurrence, and bleeding are provided in supplemental Table 4.
VTE did not cause the discontinuation of immune checkpoint inhibitor therapy, with reasons for treatment termination being radiological disease progression, immune-related adverse events, or death (including 1 confirmed and 1 suspected fatal PE and 1 fatal stroke). In 5 out 47 VTE patients (10.6%), the scheduled application of immune checkpoint inhibitor was postponed specifically due to VTE for a median of 7 days (range, 1-19 days). ATE led to treatment discontinuation in 1 patient and caused a delay in therapy in 3 patients (33.3%), with a time to therapy reinitiation of 1.6, 4.7, and 5.4 months.
Discussion
In this cohort study of patients with cancer treated with immune checkpoint inhibitors, we found high rates of VTE and ATE. The cumulative incidence estimates of our analysis indicate that approximately every 10th patient experiences VTE during immune checkpoint inhibitor therapy and every 50th patient suffers an ATE.
A substantial risk of VTE and ATE in patients treated with immune checkpoint inhibitors might be explained by several factors. These agents have been mostly approved for and used in patients with an advanced stage of disease, a known prothrombotic risk factor, and certain types of cancer with an intermediate to high risk of cancer-associated VTE and ATE such as non-small cell lung cancer.29-31 Further, immune checkpoint inhibitors are often used beyond the failure of established first-line therapy, and the level of pretreatment might contribute to the observed risk. Additionally, immune checkpoint inhibitors have led to unprecedented survival benefits, with the achievement of long-term survival despite metastatic cancer in some patients. Therefore, patients with advanced disease are at risk of thrombotic events for longer time periods compared with patients in the pre–checkpoint-inhibitor era.
Mechanistically, a systemic proinflammatory status, induced by immune checkpoint inhibitors, might enhance the prothrombotic state by activation of coagulation and platelets and impairment of fibrinolysis.32-34 Interestingly, impairment of PD-1 has been demonstrated to have a proatherogenic effect in mice.35,36 Whether the observed risk of VTE and ATE under therapy with immune checkpoint inhibitors is increased by this new type of oncologic treatment itself or just reflects the baseline risk of patients cannot be answered at this stage, as comparisons with a control group without immune checkpoint inhibitor therapy comprising similar cancer types, stages, and levels of pretreatment are currently not available. In the absence of robust comparative data from randomized clinical trials, which have tested immune checkpoint inhibitors, cohort studies of patients treated with immune checkpoint inhibitors in clinical practice can provide information on risk of thromboembolic complications.14 Currently, the only available data are from small retrospective studies, mostly focusing on patients with non-small cell lung cancer,15,16,18 which have reported an incidence of VTE ranging from 6% to 18%. These differences in previously reported rates could be explained by heterogeneity in study design, follow-up time, event definition, and most importantly characteristics of the cohorts with regard to types of cancer and risk profiles of patients, which could confound the rates in highly selected cohorts.15,16,18 Data on risk of ATE, which has been recently recognized to be also elevated in patients with cancer, are scarce.2 However, case reports describing ATE occurrence associated with immune checkpoint inhibitor application provide further basis for speculations on a potential increase in ATE risk.17,37,38
The high incidence of VTE and ATE found in our cohort highlights the need to further study clinical consequences and search for specific risk factors for VTE and ATE to better understand the association of immune checkpoint inhibitor therapy with occurrence of thromboembolic complication and potentially prevent them. Especially given the possibility of long-term treatment response and improved survival with immune checkpoint inhibitor therapy, the identification of secondary causes of morbidity and mortality, including VTE and ATE, are of utmost importance, irrespective of potential causality.
An important finding of our study is that occurrence of VTE was associated with a substantial increase in risk of mortality and poor prognosis. As the number of fatal PEs (n = 2) was not high in our study, the impact of VTE goes beyond direct VTE-related mortality and underlines the complex interrelations among the hemostatic system, VTE, and cancer on a clinical scale. Accordingly, PFS was shorter in patients who experienced VTE. Given the absence of an association of VTE and DCR, these data indicate that VTE is not related to the best radiological therapy response during immune checkpoint inhibitors, but VTE might be an indicator of treatment failure, as indicated by higher mortality and shorter time to disease progression after VTE. We could not find an association of ATE with increased risk of mortality or disease progression. However, the relatively low number of ATE events and a potential lack of power limit this analysis, and definite conclusions cannot be drawn.
Management of VTE under immune checkpoint inhibitor therapy in our cohort was heterogeneous, with considerable rates of VTE recurrence and bleeding, underlining the clinical challenges arising in this patient population. Further, the diagnosis of VTE led to a brief therapy disruption in some patients, whereas ATE frequently resulted in substantial delay in treatment and led to treatment discontinuation in one patient. In addition, fatal VTE occurred in 2 patients, and fatal ATE occurred in 1 patient.
We found an increased VTE risk in patients with a history of VTE and a trend toward increased risk with tumor stage IV, both well-recognized risk factors for cancer-associated VTE.3,29,39 However, comorbidities, performance status, sex, age, histological grade, and prior anticoagulation or antiplatelet therapy were not associated with VTE risk. Also, the Khorana score, incorporating tumor type, BMI, and laboratory parameters (hemoglobin, leukocyte, and platelet count), did not predict for VTE. This might partially be explained by differences in underlying, prothrombic risk factors between the variables in the Khorana score, designed as baseline, pretherapeutic risk predictors, and the advanced therapeutic setting in the present cohort. Furthermore, no differences in VTE risk were identified between subgroups of tumor types and among immune checkpoint inhibitory agents, except for a higher risk in patients with gynecologic cancer, an observation limited in its generalizability by the small sample size of this subgroup. For an appropriate investigation of risk factors for VTE, including biomarkers, associated with immune checkpoint inhibitors, dedicated studies with a prospective design are needed.
Our study has some other limitations. First, in order to definitively assess a potential prothrombotic effect of immune checkpoint inhibitors, data from comparative cohorts without immune checkpoint inhibitor therapy are needed. Due to uncontrollable differences in design, inclusion criteria, follow-up time, level of pretreatment, and tumor types to potentially available historical cohorts, the conduct of proper, comparative analyses was infeasible in the framework of the present study. However, we believe our observations highlight the risk of thrombotic events in patients with cancer during immune checkpoint inhibitor therapy and raise awareness. Analyses of VTE and ATE risk in patients treated with immune checkpoint inhibitors compared with a matched patient cohort may be conducted either in future cohort studies or ideally in post-hoc analysis of randomized controlled trials that have tested the efficacy of immune checkpoint inhibitors compared with standard of care in patients with cancer. Secondly, due to the retrospective cohort design, some variables, including ECOG, histological grade, and PD-L1 expression levels, were not available for all patients, and the data quality might be limited. However, as to the routine clinical oncologic care with regular clinical reassessment of patients in the setting of a tertiary care center with a special focus on the treatment of cancer patients with novel anticancer treatments, we believe that the integrity and completeness of our data, especially with respect to our primary and secondary outcome variables, are high. Furthermore, VTE and ATE events were independently adjudicated, which strengthens the validity of the data. Thirdly, as of the relatively low number of observed ATE events, no proper analysis with sufficient statistical power toward risk factors and clinical consequences of ATE under immune checkpoint inhibitor therapy could be conducted. However, the rate of ATE observed in our study is in the range of ATE rates in patients with cancer observed in recent studies.2,40,41 Despite these limitations, our study provides detailed data of a large and unselected cohort of all patients treated with immune checkpoint inhibitors over a 3-year timeframe and thereby contributes to the currently best available evidence regarding the risk of VTE and ATE associated with this novel anticancer treatment and the clinical implications of thromboembolic complications during the course of disease and therapy. The investigation of specific risk factors for VTE and ATE in our cohort was difficult, as data had to be extracted in retrospect. Therefore, future prospective studies need to specifically focus on the identification of risk factors and biomarkers to better understand and predict the risk of VTE during immune checkpoint inhibitor therapy. This might help to improve risk stratification and development of preventive strategies and risk-adapted thromboprophylaxis. Finally, we cannot provide mechanistic insight, which may explain the association of VTE occurrence and risk of mortality and early disease progression. Future investigations are required to identify specific mechanisms on the interplay among hemostasis, immune response, and cancer in the setting of immune checkpoint inhibitor therapy.
Conclusion
Patients with cancer treated with immune checkpoint inhibitors have a substantial risk of developing thromboembolic complications (both VTE and ATE). The occurrence of VTE has an impact on the clinical course and prognosis of patients receiving immune checkpoint inhibitors. Further studies are needed to better understand the risk of VTE and ATE associated with immune checkpoint inhibitors, identify risk factors predicting VTE and ATE, and improve patient care by preventing thromboembolic complications.
For original data, please contact cihan.ay@meduniwien.ac.at.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Oliver Königsbrügge for serving as chair of the adjudication committee and Barbara Helbich for her support in creating the visual abstract. The illustrations used in the visual abstract were created with BioRender.
This work was supported by the Anniversary Fund of the Austrian National Bank (grant 17828) and the Austrian Science Fund (Special Research Program [SFB] 54).
Authorship
Contribution: F.M. contributed to study design, data acquisition, data analysis, data interpretation, and manuscript writing; W.-S.E.C. and S.W. contributed to data acquisition, data analysis, data interpretation, and manuscript writing; C.H., F.T., T.F., S.Z.-M., and M.P. contributed to data interpretation and manuscript writing; M.-B.A. contributed to data acquisition and manuscript writing; I.P. contributed to study design, data interpretation, and manuscript writing; and C.A. contributed to study design, data analysis, data interpretation, and manuscript writing;
Conflict-of-interest disclosure: C.H. reports speaker honoraria from Amgen, BMS, MSD, Novartis, and Roche and advisory board participation for Amgen, Astra Zeneca, BMS, Inzyte, MSD, Novartis, Pierre Fabre, and Roche. T.F. reports honoraria from MSD, Merck Darmstadt, Roche, BMS, Accord, Sanofi, and Boehringer Ingelheim and advisory board participation for MSD, Merck Darmstadt, Amgen, Pfizer, and Sanofi. S.Z.-M. received honoraria for advisory boards and/or lectures from Boehringer Ingelheim, Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Takeda, and Pfizer and research support granted by Merck Sharp & Dohme. M.P. has received honoraria for lectures, consultation or advisory board participation from Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen. I.P. reports honoraria for lectures and advisory board meetings from Bayer AG, Boehringer Ingelheim, Daiichi Sanchyo, and BMS/ Pfizer. C.A. reports honoraria for lectures from Bayer, Daiichi Sankyo, BMS/Pfizer, and Sanofi and participation in advisory boards for Bayer, Boehringer Ingelheim, Daiichi Sankyo, and BMS/Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Cihan Ay, Clinical Division of Hematology and Hemostaseology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: cihan.ay@meduniwien.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal