TO THE EDITOR:
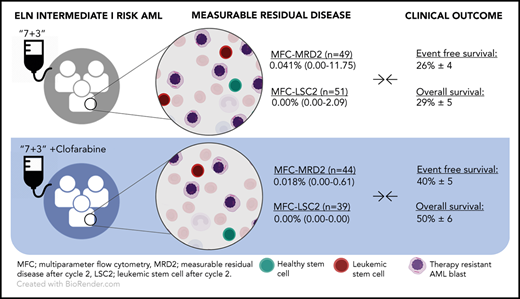
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults and is characterized by heterogeneity in cytogenetics and molecular aberrations.1 While insights in the pathogenesis and clonal landscape of AML has increased, the backbone of induction therapy has been the same for many years. In 1973, “7 + 3” cytarabine plus anthracycline chemotherapy was first described,2 achieving a 5-year overall survival (OS) of ∼40% to 50% in young patients3 and 20% to 30% in elderly patients.4 Several new (targeted) agents have been recently approved for AML,5 which look promising in a subgroup of patients,6 and some (untargeted) chemotherapeutic agents have been introduced. Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-d-arabinofuranosyladenine; a second-generation purine nucleoside analog), has shown potential benefit in young7 and older8 AML patients. However, as clofarabine was associated with a risk of severe complications in some studies9,10 but well tolerated in others,11,12 further evaluation in dosing and scheduling was warranted. In a large phase 3 study of the Dutch-Belgian Hemato-Oncology Cooperative Group–Swiss Group for Clinical Cancer Research, with ≥800 patients enrolled, a significant favorable effect of clofarabine (added to idarubicin and Ara-C) was seen in the European LeukemiaNet (ELN) intermediate-I prognostic risk subgroup13 (event-free survival, 26% ± 4% vs 40% ± 5%; Cox P = .002; OS, 29% ± 5% vs 50% ± 6%; Cox P < .001).10
One of the secondary objectives of the study was the assessment of efficacy according to measurable residual disease (MRD). Multiparameter flow cytometry (MFC)-MRD identifies leukemic cells, which can be distinguished from normal cells based on the presence of leukemia-associated immunophenotypes.14 In addition, specific antibody panels allowed MFC assessment of leukemic stem cells (LSCs).15 Samples for MFC-(LSC-)MRD detection were available for a subset of patients, and the time point of MFC-MRD assessment after 2 cycles of treatment was used for further analysis (median, 82 days after start of therapy; range, 43-252 days). See supplemental Materials and methods (available on the Blood Web site) for patient cohort information.
Here, we further analyzed the results of this MFC-(LSC-)MRD detection in this trial showing clinical benefit of clofarabine in the ELN intermediate-I risk group, demonstrating how MFC-(LSC-)MRD results after 2 cycles of chemotherapy would have predicted the beneficial effect of clofarabine at this early stage.
To qualify an assay for surrogate end point of survival benefit, the surrogate should be associated with the outcome and with the effect of the treatment and the outcome.16 A positive trial showing clinical benefit for an investigational drug is therefore crucial to assess the validity of MFC-MRD to assess effectivity of a novel drug.
MFC-MRD analysis after cycle 2 was performed in 291 patients distributed over the 4 ELN2010 risk categories.13 As clinical benefit was demonstrated in the intermediate-I ELN risk group only, we focus this analysis on this subgroup in comparison with the remaining cohort. In the intermediate-I patient group, median MFC-MRD level was 0.041% in the standard arm vs 0.018% in the clofarabine arm (n = 49 vs n = 44, respectively; P = .034) (Table 1). In the remaining cohort, median MFC-MRD level was 0.019% in the standard arm compared with 0.015% in the clofarabine arm (n = 105 vs n = 93 respectively; P = .183) (supplemental Table 2). Other time points (eg, after cycle 1 and consolidation) show no significant differences (Table 1). When using the ELN-recommended MFC-MRD cutoff (ie, ≥0.1% leukemia-associated immunophenotype cells/white blood cells),17 the prevalence of MFC-MRDpositive results seems to be lower in the clofarabine arm than in the standard arm in the intermediate-I risk group (20.4% vs 28.6%, respectively; not significant; Table 1) and in the remaining cohort (17.2% vs 23.8%, respectively; not significant; supplemental Table 2). Distinct differences in the prognostic value of MFC-MRD positivity between treatment arms were found in the intermediate-I cohort. In the cumulative incidence of relapse (CIR) analysis (Figure 1A), clofarabine-treated MFC-MRDnegative patients are less likely to relapse than MFC-MRDnegative patients treated without clofarabine (P log rank = .046). Similarly, although not significant, MFC-MRDpositive patients with clofarabine have lower CIR than MFC-MRDpositive patients without clofarabine (Figure 1A; P = .09). These observations were not found in the remaining cohort (supplemental Materials and methods).
MFC-MRD and MFC-LSC-MRD results in intermediate-I patients
| . | . | . | No. of cases evaluated . | . | |
|---|---|---|---|---|---|
| . | Control . | Clofarabine . | Control . | Clofarabine . | P . |
| MRD | |||||
| MFC-MRD1, median % (range) | 0.042 (0.00-3.51) | 0.027 (0.00-3.02) | 35 | 46 | |
| MFC-MRD2, median % (range) | 0.041 (0.00-11.75) | 0.018 (0.00-0.61) | 49 | 44 | .05 |
| MFC-MRD status | |||||
| MFC-MRD1 positive, % (n) | 31.4 (11) | 28.3 (13) | 35 | 46 | |
| MFC-MRD2 positive, % (n) | 28.6 (14) | 20.4 (9) | 49 | 44 | |
| LSCs | |||||
| MFC-LSC1, median % (range) | 0.00 (0.00-0.09) | 0.00 (0.00-0.03) | 31 | 44 | |
| MFC-LSC2, median % (range) | 0.00 (0.00-2.09) | 0.00 (0.00-0.00) | 51 | 39 | .01 |
| MFC-LSC-MRD status | |||||
| MFC-LSC1positive, % (n) | 48.4 (15) | 31.8 (14) | 31 | 44 | |
| MFC-LSC2positive, % (n) | 41.4 (21) | 20.5 (8) | 51 | 39 | |
| . | . | . | No. of cases evaluated . | . | |
|---|---|---|---|---|---|
| . | Control . | Clofarabine . | Control . | Clofarabine . | P . |
| MRD | |||||
| MFC-MRD1, median % (range) | 0.042 (0.00-3.51) | 0.027 (0.00-3.02) | 35 | 46 | |
| MFC-MRD2, median % (range) | 0.041 (0.00-11.75) | 0.018 (0.00-0.61) | 49 | 44 | .05 |
| MFC-MRD status | |||||
| MFC-MRD1 positive, % (n) | 31.4 (11) | 28.3 (13) | 35 | 46 | |
| MFC-MRD2 positive, % (n) | 28.6 (14) | 20.4 (9) | 49 | 44 | |
| LSCs | |||||
| MFC-LSC1, median % (range) | 0.00 (0.00-0.09) | 0.00 (0.00-0.03) | 31 | 44 | |
| MFC-LSC2, median % (range) | 0.00 (0.00-2.09) | 0.00 (0.00-0.00) | 51 | 39 | .01 |
| MFC-LSC-MRD status | |||||
| MFC-LSC1positive, % (n) | 48.4 (15) | 31.8 (14) | 31 | 44 | |
| MFC-LSC2positive, % (n) | 41.4 (21) | 20.5 (8) | 51 | 39 | |
MRD1/LSC1, after cycle 1; MRD2/LSC2, after cycle 2.
CIR of MFC-MRD and MFC-LSC-MRD by treatment arm. ELN intermediate-I risk patients are broken down by treatment arm (ie, standard [Stand.] vs clofarabine [Clofa] 10 mg in gray or blue, respectively) and presence or absence of MFC-MRD (A) or MFC-LSC-MRD (B) using previously published cutoffs (ie, ≥0.1% after chemotherapy cycle 2 is called MFC-MRDpositive, and ≥0.0000% after chemotherapy cycle 2 is called MFC-LSC-MRDpositive15 ). (A) MFC-MRDnegative patients treated with clofarabine have a lower incidence of relapse than MFC-MRDnegative patients in the standard arm. In parallel, MFC-MRDpositive patients with clofarabine have a lower incidence of relapse than MFC-MRDpositive patients without clofarabine. (B) Comparable to MFC-MRD, MFC-LSC-MRD shows similar results; MFC-LSC-MRDnegative patients treated with clofarabine have a distinct lower incidence of relapse than MFC-LSC-MRDnegative patients in the standard arm. In parallel, MFC-LSC-MRDpositive patients with clofarabine have a lower incidence of relapse than MFC-LSC-MRDpositive patients without clofarabine. P log-rank statistics are shown when significant.
CIR of MFC-MRD and MFC-LSC-MRD by treatment arm. ELN intermediate-I risk patients are broken down by treatment arm (ie, standard [Stand.] vs clofarabine [Clofa] 10 mg in gray or blue, respectively) and presence or absence of MFC-MRD (A) or MFC-LSC-MRD (B) using previously published cutoffs (ie, ≥0.1% after chemotherapy cycle 2 is called MFC-MRDpositive, and ≥0.0000% after chemotherapy cycle 2 is called MFC-LSC-MRDpositive15 ). (A) MFC-MRDnegative patients treated with clofarabine have a lower incidence of relapse than MFC-MRDnegative patients in the standard arm. In parallel, MFC-MRDpositive patients with clofarabine have a lower incidence of relapse than MFC-MRDpositive patients without clofarabine. (B) Comparable to MFC-MRD, MFC-LSC-MRD shows similar results; MFC-LSC-MRDnegative patients treated with clofarabine have a distinct lower incidence of relapse than MFC-LSC-MRDnegative patients in the standard arm. In parallel, MFC-LSC-MRDpositive patients with clofarabine have a lower incidence of relapse than MFC-LSC-MRDpositive patients without clofarabine. P log-rank statistics are shown when significant.
Similarly to MFC-MRD levels, lower levels of MFC-LSC-MRD were found in clofarabine-treated patients than in patients treated without clofarabine in the intermediate-I risk group (ranging from 0.00% to 2.5 × 10−4%, n = 39 vs 0.00% to 2.09%, n = 51 respectively; P = .014), while no significant difference in MFC-LSC-MRD levels were found in the remaining cohort (clofarabine vs standard, ranging from 0.00% to 3.6 × 10−3%, n = 84 vs 0.00% to 8.6 × 10−3%, n = 120, respectively) (Table 1). For MFC-LSC-MRD detection, the presence of any LSCs (<0.00001%15 ) is associated with worse outcome. In the intermediate-I group, more patients are classified MFC-LSC-MRDpositive after cycle 2 in the standard group compared with the clofarabine-treated group (41.2% vs 20.5%, respectively; P = .043). In the remaining cohort, LSC presence was found in 37.5% in the standard arm vs 28.6% in the clofarabine-treated group (P = .185; supplemental Table 2). Although not significant, within the ELN intermediate-I risk group, MFC-LSC-MRDpositive patients treated with clofarabine showed lower CIR than MFC-LSC-MRDpositive patients treated without clofarabine (Figure 1B; P = .158). Furthermore, clofarabine-treated MFC-LSC-MRDpositive patients perform similar to MFC-LSC-MRDnegative patients treated without clofarabine (Figure 1B; P = .117), which was not found in the remaining cohort (supplemental Figure 1B).
The relatively small subgroup of patients benefiting from clofarabine made it difficult to perform further meaningful analyses such as influence of molecular aberrations (FLT3 or NPM1), different time points, and multivariate analyses. The results of these analyses are shown in supplemental Figures 3-5.
In summary, this study reveals that both MFC-MRD and MFC-LSC-MRD mirror the clinical effect of clofarabine by the differences in MFC-(LSC)-MRD levels between treatment arms. However, as treatment with clofarabine is associated with enhanced toxicities, the analyses may be hindered by assessment bias, as patients going off protocol within the first 2 cycles of chemotherapy due to toxicity are not sampled for MRD detection. In addition, since treatment protocols differ among the different prognostic risk groups, the effect of allogeneic or autologous stem cell transplantation could influence results seen in event-free survival and OS (see supplemental Materials and methods).
Importantly, the proportion MFC-MRDpositive patients is dependent on the chosen cutoff and could explain why MFC-MRD positivity between treatment groups did not reach significance. Although the current ELN-recommended 0.1% cutoff is a robust cutoff in clinical trials, lower cutoffs can also render prognostic relevant MFC-MRD positivity.18,19 In large multicenter data analysis, the clinical relevance of multiple cutoffs is currently being investigated.
Despite these caveats, we show that the results of MFC-MRD and MFC-LSC-MRD after 2 cycles of chemotherapy reflect the beneficial clinical outcome of clofarabine within the ELN intermediate-I risk group. In October 2018, the Food and Drug Administration issued a draft guidance on the use of MFC-MRD for accelerated approval.20 They described that one of the requirements for surrogacy should include clinical trials in which treatment effects on the surrogate end point correspond to effects on the clinical outcome. We postulate that the difference in median MFC-(LSC-)MRD after treatments can be an interesting point to test this prospectively, as in the currently ongoing NCT03549351 trial.21
In summary, we describe a positive clinical study in which MFC-(LSC-)MRD after 2 cycles of chemotherapy reflects the effectiveness of clofarabine and the improved clinical outcome in the intermediate-I subgroup of AML patients. The results of this study point to a compelling need for further investigation of the use of MFC-MRD as instrument for surrogate short-term end point of effectiveness of new therapies.
The online version of this article contains a data supplement.
Acknowledgment
The authors thank all MRD team members of the Amsterdam UMC for sample workup and flow cytometry analysis of all samples.
Authorship
Contribution: D.H. and J.C. initiated and designed the study; D.H. and L.L.N. analyzed all data and performed statistical analysis; and D.H. and L.L.N. wrote the manuscript, which was further revised by A.v.d.L., J.J.W.M.J., G.J.O., and J.C. and reviewed by all coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacqueline Cloos, Department of Hematology, Amsterdam UMC, Vrije Universiteit Amsterdam, Cancer Center Amsterdam, The Netherlands, PO Box 7057, 1007 MB Amsterdam, The Netherlands; e-mail: j.cloos@amsterdamumc.nl.

![CIR of MFC-MRD and MFC-LSC-MRD by treatment arm. ELN intermediate-I risk patients are broken down by treatment arm (ie, standard [Stand.] vs clofarabine [Clofa] 10 mg in gray or blue, respectively) and presence or absence of MFC-MRD (A) or MFC-LSC-MRD (B) using previously published cutoffs (ie, ≥0.1% after chemotherapy cycle 2 is called MFC-MRDpositive, and ≥0.0000% after chemotherapy cycle 2 is called MFC-LSC-MRDpositive15). (A) MFC-MRDnegative patients treated with clofarabine have a lower incidence of relapse than MFC-MRDnegative patients in the standard arm. In parallel, MFC-MRDpositive patients with clofarabine have a lower incidence of relapse than MFC-MRDpositive patients without clofarabine. (B) Comparable to MFC-MRD, MFC-LSC-MRD shows similar results; MFC-LSC-MRDnegative patients treated with clofarabine have a distinct lower incidence of relapse than MFC-LSC-MRDnegative patients in the standard arm. In parallel, MFC-LSC-MRDpositive patients with clofarabine have a lower incidence of relapse than MFC-LSC-MRDpositive patients without clofarabine. P log-rank statistics are shown when significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/137/12/10.1182_blood.2020007150/1/m_bloodbld2020007150f1.png?Expires=1769179937&Signature=Tc3-CvK915ZSFJmQEo2nBr36bOqafCGF4AnpuzwPoaYshzSth0dPgG7vP4GaZerE~qX8ZVU8MszV~mSHVbX7SgsXSjdb-7V9GxkPcNGU8BKI0YtYs~7F-q69WQWYEazX67wzMxpc7-sMehZhWZX4iftHxgyAanIMWCqQhKVMf7Fe23YS7PNyKKy6b2-NmrQpKSKy4jiZiD056pM5vYeMwwksnmveOhFvqf6UMcCRGrllh6q9SYy4Xd0tecTG00WD9xyHwdKw1sPM1guEloCRQDUqKBrNtxR06bVM0t0Z8se1X-OKk7Kw9RAdnvEroaXU6d6Zvd79o9YPcygL3ZdcZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)