Key Points
Permissive HLA-DPB1 mismatches in HCT are characterized by limited immunopeptidome divergence, mirrored by alloreactive TCR diversity.
The peptide editor HLA-DM plays a key role in harnessing T-cell alloreactivity to permissive HLA-DP by restricting its peptide repertoire.
Abstract
In hematopoietic cell transplantation (HCT), permissive HLA-DPB1 mismatches between patients and their unrelated donors are associated with improved outcomes compared with nonpermissive mismatches, but the underlying mechanism is incompletely understood. Here, we used mass spectrometry, T-cell receptor-β (TCRβ) deep sequencing, and cellular in vitro models of alloreactivity to interrogate the HLA-DP immunopeptidome and its role in alloreactive T-cell responses. We find that permissive HLA-DPB1 mismatches display significantly higher peptide repertoire overlaps compared with their nonpermissive counterparts, resulting in lower frequency and diversity of alloreactive TCRβ clonotypes in healthy individuals and transplanted patients. Permissiveness can be reversed by the absence of the peptide editor HLA-DM or the presence of its antagonist, HLA-DO, through significant broadening of the peptide repertoire. Our data establish the degree of immunopeptidome divergence between donor and recipient as the mechanistic basis for the clinically relevant permissive HLA-DPB1 mismatches in HCT and show that permissiveness is dependent on HLA-DM–mediated peptide editing. Its key role for harnessing T-cell alloreactivity to HLA-DP highlights HLA-DM as a potential novel target for cellular and immunotherapy of leukemia.
Introduction
Limited T-cell alloreactivity sufficient for graft-versus-leukemia effects, but not for severe graft-versus-host disease (GVHD), is a holy grail in hematopoietic cell transplantation (HCT).1 For HLA-DPB1, mismatched in >80% unrelated donor (UD)–recipient pairs, this can be achieved by functional matching for T-cell epitope (TCE) groups.2 Permissive HLA-DPB1 TCE mismatches are associated with more limited in vitro T-cell alloreactivity3 and significantly lower risks of mortality and relapse after UD-HCT compared with their nonpermissive and matched counterparts, respectively,4,5 prompting inclusion of the HLA-DP TCE algorithm into international donor selection guidelines.6 However, the mechanisms underlying these observations are insufficiently understood. We have shown that peptide contact residues in HLA-DPB1 are important for alloreactivity7 and that binding motifs and peptide composition correlate with TCE group assignment.8 We therefore hypothesized that immunopeptidome divergence between mismatched HLA-DPB1 and peptide editing by the endosomal chaperone HLA-DM9,10 might regulate TCE permissiveness.
Study design
Detailed materials and methods are available as supplemental Methods. B-lymphoblastoid cell lines (BLCL), as well as HeLa cells expressing invariant chain (Ii), CD80, and individual HLA-DP allotypes (HeLa-DP) with or without HLA-DM, were used to characterize HLA-DP–bound peptides by tandem mass spectrometry.8,11 HeLa cells were also used for in vitro stimulation of CD4+ T cells from healthy individuals or patients after HLA-DP–mismatched UD-HCT (supplemental Figure 1 and supplemental Table 1, available at the Blood Web site). Alloreactive T-cell receptor β (TCRβ) clonotype composition and diversity was characterized by next-generation sequencing.12
Results and discussion
Limited peptide divergence is a hallmark of permissive HLA-DPB1 mismatches
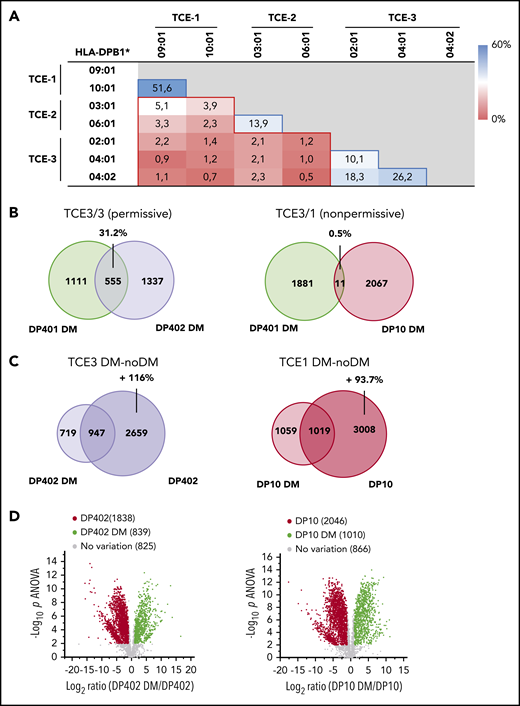
Donor–recipient mismatches for HLA-DPB1 alleles from the same TCE group are considered permissive, while those across different TCE groups are nonpermissive (supplemental Figure 2). We performed pairwise comparisons of the immunopeptidomes of representative frequent HLA-DP allotypes from the 3 TCE groups on BLCL. Overlaps were significantly higher between immunopeptidomes of permissive compared with nonpermissive allotype combinations (Figure 1A). To account for possible impacts of non-HLA gene polymorphisms, we confirmed these findings in the same cellular background using HeLa-DP (supplemental Figures 3 and 4A) for 2 representative mismatch combinations between HLA-DP401 and permissive HLA-DP402 or nonpermissive HLA-DP10 (Figure 1B). These data suggest that immunopeptidome divergence could help redefine certain debated permissive or nonpermissive HLA-DPB1 mismatch combinations8 and might represent an appealing new approach for characterizing permissiveness also for other HLA loci.
Immunopeptidome overlaps of HLA-DP allotypes from different TCE groups and their dependency on HLA-DM. (A) Percentage of unique peptides shared between the HLA-DP immunopeptidomes from BLCLs expressing the indicated paired allotypes, characterized as described in van Balen et al.8 Data from at least 2 biological and 3 technical replicates were coprocessed for the analysis. Blue- and red-lined boxes represent permissive and nonpermissive allotype combinations with a mean ± standard deviation (SD) overlap percentage of 24.0 ± 16.5 and 2.0 ± 1.3, respectively (P < .001 in 2-tailed unpaired t test). Peptide repertoire overlaps between ≤3 different BLCLs expressing the same HLA-DPB1 allele were determined for HLA-DPB1*02:01, 03:01, and 04:01 and had a mean of 41.4% ± 8.1%. (B) Peptide overlap in permissive (DP401 vs DP402) or nonpermissive (DP401 vs DP10) HLA-DP allotype combinations determined in the same cellular background of transduced HeLa cells in the presence of HLA-DM. Numbers indicate unique or shared peptides, the latter with its percentage relative to the combined data set. Data from at least 2 biological and 3 technical replicates were coprocessed for the analysis. (C) Overlap between peptides retrieved from DP402 or DP10 expressed by HeLa cells in the presence or absence of HLA-DM. Numbers indicate unique or shared peptides and the percentage of numerical inflation of the peptide repertoire in the absence of HLA-DM relative to its presence. (D) Volcano plots showing unique peptides significantly enriched in the presence (green) or absence (red) of HLA-DM for DP402 and DP10. Significant enrichment was assigned to peptides with at least twofold variation of relative abundance in the presence vs absence of HLA-DM and significant (P < .01) in false discovery rate–adjusted 1-way analysis of variance (ANOVA).
Immunopeptidome overlaps of HLA-DP allotypes from different TCE groups and their dependency on HLA-DM. (A) Percentage of unique peptides shared between the HLA-DP immunopeptidomes from BLCLs expressing the indicated paired allotypes, characterized as described in van Balen et al.8 Data from at least 2 biological and 3 technical replicates were coprocessed for the analysis. Blue- and red-lined boxes represent permissive and nonpermissive allotype combinations with a mean ± standard deviation (SD) overlap percentage of 24.0 ± 16.5 and 2.0 ± 1.3, respectively (P < .001 in 2-tailed unpaired t test). Peptide repertoire overlaps between ≤3 different BLCLs expressing the same HLA-DPB1 allele were determined for HLA-DPB1*02:01, 03:01, and 04:01 and had a mean of 41.4% ± 8.1%. (B) Peptide overlap in permissive (DP401 vs DP402) or nonpermissive (DP401 vs DP10) HLA-DP allotype combinations determined in the same cellular background of transduced HeLa cells in the presence of HLA-DM. Numbers indicate unique or shared peptides, the latter with its percentage relative to the combined data set. Data from at least 2 biological and 3 technical replicates were coprocessed for the analysis. (C) Overlap between peptides retrieved from DP402 or DP10 expressed by HeLa cells in the presence or absence of HLA-DM. Numbers indicate unique or shared peptides and the percentage of numerical inflation of the peptide repertoire in the absence of HLA-DM relative to its presence. (D) Volcano plots showing unique peptides significantly enriched in the presence (green) or absence (red) of HLA-DM for DP402 and DP10. Significant enrichment was assigned to peptides with at least twofold variation of relative abundance in the presence vs absence of HLA-DM and significant (P < .01) in false discovery rate–adjusted 1-way analysis of variance (ANOVA).
HLA-DM–mediated editing restricts HLA-DP peptide diversity
HLA-DM is an endosomal chaperone generally coexpressed with HLA class II, which facilitates the removal of cleaved invariant chain peptide (CLIP) and loading of high-affinity peptides. HLA-DM function can be antagonized or altered by HLA-DO in certain B-lineage cells.9,10 We found that the absence of HLA-DM led to an approximately twofold inflation in the number and abundance of peptides retrieved from HLA-DP402 and HLA-DP10 (Figure 1C-D), with concomitant broadening of their cellular component sources but no change in their length or motif (supplemental Figure 4B-D). This is in contrast to HLA-DR, where the absence of HLA-DM leads to changes in the peptide motif and preferential CLIP binding.13,14 Thus, CLIP might interfere with HLA-DR–restricted presentation of tumor antigens, possibly explaining previous observations that high CLIP expression levels by acute myeloid leukemia are associated with poor clinical outcome.15 Although we found more CLIP peptides in HLA-DP10 than in HLA-DP402, consistent with previous observations,16 CLIP accounted only for <10% of the total peptide pool in the absence of HLA-DM and even less in its presence (supplemental Figure 4E). Thus, HLA-DP maintains a diverse peptide repertoire in the absence of HLA-DM, making it an appealing target for cellular therapy.17
Limited T-cell alloreactivity to permissive HLA-DP TCE mismatches is dependent on HLA-DM
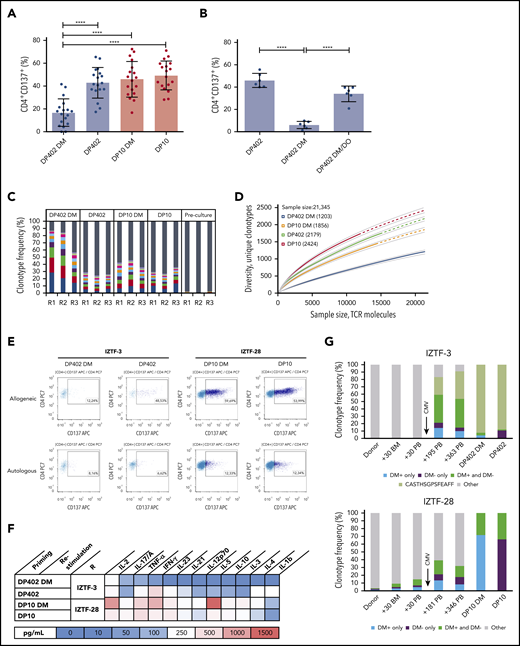
Consistent with previous findings,3 CD4+ T-cell activation and proinflammatory Th1/Th17 cytokine production in healthy individuals was lower against permissive than against nonpermissive HLA-DP in the presence of HLA-DM (Figure 2A; supplemental Figure 5A-B). Strikingly, T-cell alloreactivity to permissive HLA-DP402, but not to nonpermissive HLA-DP10, increased significantly in the absence of HLA-DM. T cells expanded against HLA-DP in the absence of HLA-DM were not efficiently stimulated by the same allotype in the presence of HLA-DM, whereas the opposite was true for those raised against the allotype in the presence of HLA-DM (supplemental Figure 6), suggesting that most allopeptides in this setting are sensitive to HLA-DM–mediated editing. Quenching of the alloresponse by HLA-DM could be reversed by its antagonist HLA-DO (Figure 2B; supplemental Figure 5C). Permissive alloresponses in the presence of HLA-DM showed lower clonotypic TCRβ diversity with higher cumulative frequency of top-10 CDR3 rearrangements, lower number of unique clonotypes at different sampling depths, and lower TCR-Vβ responsiveness, compared with the absence of HLA-DM or with nonpermissive alloresponses (Figure 2C-D; supplemental Figure 7A-B). To obtain a first insight into these mechanisms in the clinical setting, we investigated 2 patients after HCT from UDs with a permissive HLA-DP402 or a nonpermissive HLA-DP10 mismatch in the graft-versus-host direction, respectively (supplemental Table 1). At 1 year after HCT, isolated full donor chimeric CD4+ T cells from these patients showed markedly lower CD137 T-cell activation and cytokine responses in the permissive compared with the nonpermissive setting, and quenching of the former by HLA-DM (Figure 2E-F). The permissive alloresponse was dominated by an expanded TCRβ clonotype both in the presence and absence of HLA-DM, which emerged between day +30 and day +195 after transplantation, concomitant with high-titer cytomegalovirus (CMV) reactivation (Figure 2G). The clonotypic composition of the nonpermissive alloresponse was more diverse and less similar between the HLA-DM positive or negative condition. Nonpermissive clonotypes also expanded in vivo, after CMV reactivation, although to a lesser extent, in line with previously reported profound effects of CMV on post-HCT immune reconstitution18 (Figure 2G).
HLA-DM–mediated modulation of alloreactive T-cell responses to HLA-DP in healthy individuals and transplanted patients. (A-B) Activation of CD4+ T cells from healthy HLA-DP401+ individuals in response to permissive HLA-DP402 (blue dots) or nonpermissive HLA-DP10 (red dots). Shown is the percentage of activated (CD137+) CD4+ T cells after restimulation with HeLa-DP as indicated (see also supplemental Figure 1). Values are reported after subtraction of background activation against HeLa-DP expressing the autologous allotype in the presence of HLA-DM. Statistical comparisons by 1-way ANOVA. ***P < .001, ****P < .0001. (A) CD4+ T cells from healthy individuals (n = 18) were cocultured and restimulated with the same allogeneic HLA-DP/HLA-DM combination as indicated. Mean ± SD percentage autologous background was 7.63 ± 4.65. (B) CD4+ T cells from healthy individuals (n = 6) were cocultured with HLA-DP402 without HLA-DM and restimulated with allogeneic HLA-DP/HLA-DM/HLA-DO combinations as indicated. Mean ± SD percentage autologous background was 16.0 ± 9.6. (C-D) The alloreactive TCRβ repertoire of activated CD4+CD137+ T cells in representative healthy individuals from panel A, characterized by next-generation sequencing. (C) Relative frequency of top-10 or background clonotypes in 3 responders (R1-R3), indicated as colored or gray bars, respectively. (D) Rarefaction curves of TCRβ clonotypes in R1 (R2 and R3 in supplemental Figure 7). Curves are interpolated from 0 to the size of each sample (solid lines) and extrapolated to the size of the largest sample (dashed lines). Numbers in the legend indicate unique clonotypes in each repertoire at the maximum sample size. (E-G) CD4+ T-cell activation and alloreactive TCRβ repertoire in transplanted patients after HCT from UDs mismatched for permissive HLA-DP402 (IZTF-3) or nonpermissive HLA-DP10 (IZTF-28). Full donor CD4+ T cells were isolated from the patients’ peripheral blood at days 363 and 346, respectively (supplemental Table 1), and used for coculture and restimulation with HeLa-DP expressing the mismatched allotype in the presence or absence of HLA-DM. (E) Fluorescence activated cell sorting plots of CD137 activation assays. Specific percentages of alloresponses with or without HLA-DM was 3.87 and 41.91 (IZTF-3) or 41.82 and 47.84 (IZTF-28). (F) Heatmap of cytokine production. Background response to autologous HLA-DP was <100 pg/mL. (G) Alloreactive CD4+ TCRβ clonotypes after in vitro culture (2 rightmost bars) were traced ex vivo in peripheral blood or bone marrow from the patients or their respective UD at the indicated time points. Shown is the cumulative frequency of TCRβ clonotypes found only in the presence (blue) or absence (purple) of HLA-DM, or in both (green), with an expanded clonotype in IZTF-3 in lighter green. Both patients experienced early CMV reactivation as indicated. BM, bone marrow; IL, interleukin; PB, peripheral blood.
HLA-DM–mediated modulation of alloreactive T-cell responses to HLA-DP in healthy individuals and transplanted patients. (A-B) Activation of CD4+ T cells from healthy HLA-DP401+ individuals in response to permissive HLA-DP402 (blue dots) or nonpermissive HLA-DP10 (red dots). Shown is the percentage of activated (CD137+) CD4+ T cells after restimulation with HeLa-DP as indicated (see also supplemental Figure 1). Values are reported after subtraction of background activation against HeLa-DP expressing the autologous allotype in the presence of HLA-DM. Statistical comparisons by 1-way ANOVA. ***P < .001, ****P < .0001. (A) CD4+ T cells from healthy individuals (n = 18) were cocultured and restimulated with the same allogeneic HLA-DP/HLA-DM combination as indicated. Mean ± SD percentage autologous background was 7.63 ± 4.65. (B) CD4+ T cells from healthy individuals (n = 6) were cocultured with HLA-DP402 without HLA-DM and restimulated with allogeneic HLA-DP/HLA-DM/HLA-DO combinations as indicated. Mean ± SD percentage autologous background was 16.0 ± 9.6. (C-D) The alloreactive TCRβ repertoire of activated CD4+CD137+ T cells in representative healthy individuals from panel A, characterized by next-generation sequencing. (C) Relative frequency of top-10 or background clonotypes in 3 responders (R1-R3), indicated as colored or gray bars, respectively. (D) Rarefaction curves of TCRβ clonotypes in R1 (R2 and R3 in supplemental Figure 7). Curves are interpolated from 0 to the size of each sample (solid lines) and extrapolated to the size of the largest sample (dashed lines). Numbers in the legend indicate unique clonotypes in each repertoire at the maximum sample size. (E-G) CD4+ T-cell activation and alloreactive TCRβ repertoire in transplanted patients after HCT from UDs mismatched for permissive HLA-DP402 (IZTF-3) or nonpermissive HLA-DP10 (IZTF-28). Full donor CD4+ T cells were isolated from the patients’ peripheral blood at days 363 and 346, respectively (supplemental Table 1), and used for coculture and restimulation with HeLa-DP expressing the mismatched allotype in the presence or absence of HLA-DM. (E) Fluorescence activated cell sorting plots of CD137 activation assays. Specific percentages of alloresponses with or without HLA-DM was 3.87 and 41.91 (IZTF-3) or 41.82 and 47.84 (IZTF-28). (F) Heatmap of cytokine production. Background response to autologous HLA-DP was <100 pg/mL. (G) Alloreactive CD4+ TCRβ clonotypes after in vitro culture (2 rightmost bars) were traced ex vivo in peripheral blood or bone marrow from the patients or their respective UD at the indicated time points. Shown is the cumulative frequency of TCRβ clonotypes found only in the presence (blue) or absence (purple) of HLA-DM, or in both (green), with an expanded clonotype in IZTF-3 in lighter green. Both patients experienced early CMV reactivation as indicated. BM, bone marrow; IL, interleukin; PB, peripheral blood.
Overall, our data suggest that an indirect effect of thymic education19 efficiently prunes out T cells recognizing shared peptides presented in structurally similar, permissive HLA-DP molecules. The resulting repertoire of alloreactive TCRβ clonotypes responds more readily to peptides emerging in structurally divergent, nonpermissive HLA-DP mismatches, or in permissive HLA-DP mismatches via inhibition of HLA-DM–mediated editing. Besides HLA immunopeptidome divergence, TCRβ clonotype diversity could therefore be a marker of permissiveness, as previously suggested for solid organ transplantation.20 In vivo, this model of permissiveness might be complementary to algorithms associating HLA expression levels with the risks of GVHD after UD-HCT.21-23 Thus, limited numbers of unique peptides in permissive mismatch combinations might be more immunogenic when presented by highly expressed HLA allotypes. This is consistent with recent observations that accounting for high expression mismatches significantly improved GVHD associations in HLA-DPB1 TCE permissive, but not nonpermissive, pairs23,24 . Our data further suggest that HLA-DM polymorphisms associated with potentially altered editing function, occurring with frequencies between 0.01 and 0.7 in different world populations,25 might cooperate with TCE groups and/or expression levels in determining the functional impact of HLA-DP disparity. HLA-DM typing of clinical cohorts and functional characterization of frequent HLA-DM variants is warranted to better understand the relevance of these complex interactions for HCT outcome. Finally, our findings open new potential strategies for exploiting allogeneic HLA-DP–restricted peptide antigens and their editing by HLA-DM as innovative targets for cellular immunotherapy of leukemia via vaccination, selection of HLA-DM status–specific T-cell products or receptors, or immunopeptidome modulation via targeted pharmacologic or gene-editing interventions.17
The immunopeptidomics and TCRβ immunosequencing data reported in this article have been deposited in the Proteomic Identification Database Archive (accession number PXD017154) and the ImmuneACCESS Database (Adaptive Biotechnologies; ImmuneACCESS DOI https://doi.org/10.21417/TM2020I), respectively.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mirko Trilling (Essen) and James P. Di Santo (Paris) for critically reading the manuscript. The Cell Sorting Facility (IMCES) and the Biochip Laboratory of UK-Essen are gratefully acknowledged for their support with the relevant experiments.
This work was supported by the Deutsche Forschungsgemeinschaft (grant DFG FL 843/1-1) (K.F.), the Deutsche José Carreras Leukämie Stiftung (grants DJCLS R 15-02 and DJCLS 20R/2019) (K.F.), the Joseph-Senker Stiftung (K.F.), the Deutsche Knochenmarkspendedatei (grant DKMS-SLS-MHG-2018-01) (P.C.), the ZonMw (Investment Grant NOW Medium 91116004) (P.A.v.V.), and P.U.R.E. (Protein Research Unit Ruhr within Europe), Ministry of Innovation, Science and Research of North-Rhine Westphalia, Germany (B.S.).
Authorship
Contribution: K.F., E.A.-B., and P.C. designed the study; T.M., P.C., E.A.-B., and K.F. wrote the manuscript; T.M., P.C., M.M., M.K., D.A.M., W.C., P.A.v.V., G.H., S.E.L., and E.A.-B. performed experiments; A.T.T. provided clinical patient data; P.A.H. provided access to healthy donor material; P.v.B., A.M.W., M.G., P.A.H., B.S., D.W.B, and J.H.F.F. provided significant advice throughout the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katharina Fleischhauer, Institute for Experimental Cellular Therapy, University Hospital Essen, Essen, Germany; e-mail: katharina.fleischhauer@uk-essen.de.
REFERENCES
Author notes
T.M. and P.C. contributed equally to this study.
E.A.-B. and K.F. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal