TO THE EDITOR:
Chemoimmunotherapy represents the standard frontline treatment for patients with low-grade advanced-stage follicular lymphoma (FL) and high tumor burden.1,2
More recently, the combination of lenalidomide, an oral immunomodulatory agent,3 and rituximab (R2) has been investigated in 3 phase 2 studies (NCT01307605, NCT00695786, NCT01145495) and a randomized phase 3 trial (NCT01476787, NCT01650701) named RELEVANCE.4-7 In the latter, 1030 patients with advanced-stage FL were randomized to receive frontline R2 or chemoimmunotherapy. Although the primary end point was progression-free survival (PFS), in light of the limited median follow-up (3 years), results for the coprimary end point (complete remission [CR] at 30 months) were reported; R2 had similar efficacy to chemoimmunotherapy.7 As such, R2 has been used by several clinicians as an alternative frontline treatment option to chemoimmunotherapy in select patients. Long-term follow-up data regarding the toxicity and efficacy of this regimen have yet to be presented and are highly needed.
This is an analysis of patients with grade 1 or 2 and stage III or IV FL receiving frontline treatment with R2 on a phase 2 clinical trial (NCT00695786) at The University of Texas MD Anderson Cancer Center between July 2008 and April 2012 (data cutoff date: 16 May 2020).
The 1999 International Working Group criteria for non-Hodgkin lymphomas were prospectively used for response assessment.8 The Follicular Lymphoma Internal Prognostic Index (FLIPI), FLIPI-2, and the PRIMA-Prognostic Index (PRIMA-PI) were assessed as previously described.9-11 The 2014 Lugano classification was used to retrospectively define complete response.12 The study was approved by the Institutional Review Board of MD Anderson Cancer Center and conducted in accordance with our institutional guidelines and the principles of the Declaration of Helsinki. Two treatment schedules were used in the study: schedule A and schedule B (details are provided in supplemental Materials, available on the Blood Web site). A landmark analysis, using median time to achievement of CR as landmark time, was performed for PFS comparisons (additional details about statistical methods are provided in supplemental Materials).
Seventy-nine patients were included in the study. Baseline characteristics are shown in Table 1.
Patients’ baseline characteristics (N = 79)
| Characteristic . | n (%) . |
|---|---|
| Age, y | |
| <60 | 43 (54) |
| ≥60 | 36 (46) |
| Ethnicity | |
| White | 72 (91) |
| Non-white | 5 (6) |
| Unknown | 2 (3) |
| Sex | |
| Female | 39 (49) |
| Male | 40 (51) |
| Hemoglobin, g/dL | |
| ≥12 | 75 (95) |
| <12 | 4 (5) |
| β2-microglobulin | |
| Low-normal | 51/68 (75) |
| Elevated | 17/68 (25) |
| Lactate dehydrogenase | |
| Low-normal | 73/75 (97) |
| Elevated | 2/75 (3) |
| Bone marrow | |
| Not involved | 44 (56) |
| Involved | 35 (44) |
| B symptoms | |
| Absent | 69 (87) |
| Present | 10 (13) |
| Ann Arbor stage | |
| III | 35 (44) |
| IV | 44 (56) |
| Involved nodal areas, n | |
| <5 | 31 (39) |
| ≥5 | 48 (61) |
| Size of largest lymph node, cm | |
| <6 | 71 (90) |
| ≥6 | 8 (10) |
| Extranodal disease | |
| Absent | 50 (63) |
| Present | 29 (37) |
| FLIPI score | |
| Low | 31 (39) |
| Intermediate | 34 (43) |
| High | 14 (18) |
| FLIPI-2 score | |
| Low | 10 (12) |
| Intermediate | 51 (65) |
| High | 18 (23) |
| PRIMA-PI | |
| Low | 42 (53) |
| Intermediate | 32 (41) |
| High | 5 (6) |
| SUVmax | |
| ≤10 | 40/76 (53) |
| >10 | 36/76 (47) |
| Characteristic . | n (%) . |
|---|---|
| Age, y | |
| <60 | 43 (54) |
| ≥60 | 36 (46) |
| Ethnicity | |
| White | 72 (91) |
| Non-white | 5 (6) |
| Unknown | 2 (3) |
| Sex | |
| Female | 39 (49) |
| Male | 40 (51) |
| Hemoglobin, g/dL | |
| ≥12 | 75 (95) |
| <12 | 4 (5) |
| β2-microglobulin | |
| Low-normal | 51/68 (75) |
| Elevated | 17/68 (25) |
| Lactate dehydrogenase | |
| Low-normal | 73/75 (97) |
| Elevated | 2/75 (3) |
| Bone marrow | |
| Not involved | 44 (56) |
| Involved | 35 (44) |
| B symptoms | |
| Absent | 69 (87) |
| Present | 10 (13) |
| Ann Arbor stage | |
| III | 35 (44) |
| IV | 44 (56) |
| Involved nodal areas, n | |
| <5 | 31 (39) |
| ≥5 | 48 (61) |
| Size of largest lymph node, cm | |
| <6 | 71 (90) |
| ≥6 | 8 (10) |
| Extranodal disease | |
| Absent | 50 (63) |
| Present | 29 (37) |
| FLIPI score | |
| Low | 31 (39) |
| Intermediate | 34 (43) |
| High | 14 (18) |
| FLIPI-2 score | |
| Low | 10 (12) |
| Intermediate | 51 (65) |
| High | 18 (23) |
| PRIMA-PI | |
| Low | 42 (53) |
| Intermediate | 32 (41) |
| High | 5 (6) |
| SUVmax | |
| ≤10 | 40/76 (53) |
| >10 | 36/76 (47) |
All patients had low-grade FL (grade 1-2); Ki67 was assessed in 18 patients, and it was ≥40% in 3 (17%).
The median number of provided cycles was 6 (range, 1-12); 49 patients received 1 to 6 cycles (47 with schedule A, 2 with schedule B), and 30 patients received 7 to 12 cycles (28 with schedule B, 2 with schedule A). More patients who received 7 to 12 cycles of treatment had high baseline β2-microglobulin (46% vs 14%; P = .007), >5 lymph node sites (80% vs 49%; P = .009), and high FLIPI-2 score (37% vs 14%; P = .03) compared with those who received 1 to 6 cycles of treatment.
Median dose of lenalidomide was 20 mg (range, 5-20), and 24 (30%) patients required a dose reduction because of toxicity. Seven (9%) patients discontinued treatment before completion, after a median time of 4 months (range, 1-10 months): 4 because of toxicity (arterial thrombosis in 2, respiratory failure in 1, and skin rash in 1) and 3 because of progression.
Overall, 40 (51%) patients experienced grade ≥3 treatment-related toxicity (supplemental Materials). The incidence of grade 3-4 neutropenia was significantly lower in patients receiving 1 to 6 cycles of treatment compared with those who received 7 to 12 cycles (22% vs 70%, P < .001).
Best response was evaluable in 76 patients; 3 patients discontinued treatment because of toxicity before first response assessment. In an intention-to-treat analysis, overall response rate was achieved in 75 of 79 (95%) patients, and CR was achieved in 68 of 79 (86%) patients, both after a median of 6 months (range, 3-18 months). Of interest, CR was achieved in 39 (49%) patients after 3 cycles and in 64 (81%) patients after 6 cycles. The only factor associated with achievement of CR on univariate analysis was sex (CR rate was 97% for females vs 75% for males, P = .005). No difference in CR rate was observed based on dose reduction (P = .25) or number of provided cycles (P = 1).
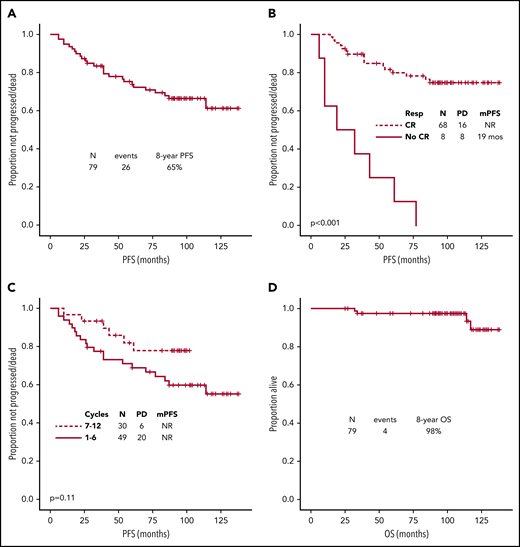
After a median follow-up of 103 months (95% confidence interval [CI], 98-108), 26 (33%) patients had progressed/died, median PFS was not reached, and the 8-year PFS rate was 65% (Figure 1A). Among 23 patients who relapsed after frontline R2, 10 were treated with chemoimmunotherapy, 4 were treated with single-agent rituximab, 2 were retreated with R2, 4 were placed on a clinical trial, and 3 were observed. None received autologous stem cell transplant as first salvage. The only baseline characteristic associated with shorter PFS was non-white ethnicity (39 months vs not reached, P < .001). Lack of achievement of CR (by 2014 Lugano criteria) as best response was associated with longer median PFS on univariate analysis (not reached vs 19 months, P < .001) (Figure 1B). The association between lack of achievement of CR and shorter median PFS was also confirmed on a landmark analysis, using median time to achievement of CR (6 months) as landmark time (P = .001). No association between PFS and lenalidomide dose reduction (P = .72) or number of cycles of treatment (P = .11) was observed (Figure 1C).
Survival. (A) PFS for all patients. (B) PFS by achievement of CR as best response. (C) PFS by number of cycles. (D) Overall survival (OS). At the most recent follow-up, transformation was reported in only 1 (1%) patient, after 30 months from initiation of treatment. Secondary cancers (excluding transformation) were diagnosed in 8 (10%) patients after a median of 59 months (range, 3-105). These included melanoma (2), pancreatic adenocarcinoma (1), esophageal adenocarcinoma (1), therapy-related acute myeloid leukemia (1), basal cell carcinoma of the skin (1), smoldering multiple myeloma (1), and renal cell carcinoma (1). Causes of death included secondary cancers in 2 patients (metastatic pancreatic adenocarcinoma and relapsed/refractory acute myeloid leukemia), unrelated comorbid health conditions in 1 patient, and progressive disease in 1 patient. mPFS, median PFS; NR, not reached; PD, progressive disease/death; Resp, response.
Survival. (A) PFS for all patients. (B) PFS by achievement of CR as best response. (C) PFS by number of cycles. (D) Overall survival (OS). At the most recent follow-up, transformation was reported in only 1 (1%) patient, after 30 months from initiation of treatment. Secondary cancers (excluding transformation) were diagnosed in 8 (10%) patients after a median of 59 months (range, 3-105). These included melanoma (2), pancreatic adenocarcinoma (1), esophageal adenocarcinoma (1), therapy-related acute myeloid leukemia (1), basal cell carcinoma of the skin (1), smoldering multiple myeloma (1), and renal cell carcinoma (1). Causes of death included secondary cancers in 2 patients (metastatic pancreatic adenocarcinoma and relapsed/refractory acute myeloid leukemia), unrelated comorbid health conditions in 1 patient, and progressive disease in 1 patient. mPFS, median PFS; NR, not reached; PD, progressive disease/death; Resp, response.
Progression of disease within 24 months (POD24) was observed in 10 (13%) patients. The only factor significantly associated with POD24 was lack of achievement of CR as best response (POD24 rate 50% vs 7%, P = .005). No association between POD24 and lenalidomide dose reduction (P = 1) or number of cycles of treatment (P = .30) was observed. No patient with POD24 had died at the most recent follow-up.
At the most recent follow-up, 1 patient was lost to follow-up, and 4 (5%) patients had died. Median overall survival (OS) had not been reached, and 8-year OS rate was 98% (Figure 1D). Therefore, no association with baseline characteristics could be assessed.
In this study, CR rate and PFS (8-year PFS rate 65%) were independent of traditional baseline prognostic factors. In this regard, novel prognostic and predictive models dissecting the role of the tumor microenvironment in the immunotherapy era are desperately needed to select FL patients who are more likely to respond to R2.13,14 CR rate and PFS were also independent of baseline maximum standardized uptake value (SUVmax), which may be a surrogate marker for more aggressive biology.15,16 The optimal frontline treatment of these patients has yet to be identified. In the present study, outcomes were independent of lenalidomide dose reductions and the number of cycles of treatment. It is important to note that multiple treatment schedules have been used in the 3 phase 2 trials and the 1 randomized phase 3 study investigating the efficacy and safety of frontline R2 in patients in FL. Although, based on the latter, the standard schedule consists of 18 months of treatment, with dose reduction allowed only in case of CR after 6 cycles or renal insufficiency, other investigated schedules have shown similar efficacy for shorter-duration regimens, as long as ≥6 months of treatment are provided.7-9 As shown in this and other studies, although lenalidomide is an immunotherapy, it can be associated with neutropenia (particularly if prolonged treatment is needed), fatigue, skin rash, and occasional disimmune complications. In this regard, in this study, no difference in CR rate, PFS, or OS was observed when comparing a shorter-duration regimen with a longer-duration regimen, with the advantage of a lower rate of treatment-related toxicities, such as neutropenia. Although this needs to be confirmed prospectively, the identification of biomarkers to select FL patients who are most likely to respond to frontline R2 will also help to determine the ideal dosing and schedule in the future.
The only factor associated with prolonged PFS and shorter POD24 in the current study was achievement of CR as best response to R2, similarly to what also reported for other regimens.17-21 Multiple attempts have been done over the last years to improve on the efficacy of frontline R2, either by substituting rituximab for obinutuzumab, or by adding a third agent, such as ibrutinib.22,23 New combinations in the future will provide novel and more effective treatment options for these patients.24-26 Of interest, in the present study, despite a POD24 of 13% and median follow-up of 8 years, no significant association between the former and OS could be assessed, in light of the paucity of reported events (4 deaths).
Long- and short-term toxicity data collected from this study were encouraging. Although 2 (2.5%) patients discontinued treatment because of arterial thrombosis, in a meta-analysis of 29 independent cohorts, including 18 018 patients with B-cell non-Hodgkin lymphoma, 1.1% of individuals experienced an arterial thrombotic event within 3 years, suggesting that its risk may be increased by patient and disease-related factors, in addition to the selected treatment regimen.27 Similarly, in a retrospective registry study including 11 055 patients with FL who survived for >1 year after initial diagnosis, over a 14-year observation period, 685 (6%) developed secondary cancers (other than transformation), suggesting that the 10% incidence observed in our study may also be driven by patient- and disease-specific biological factors.28
In our study, 4 (5%) patients died after a median follow-up of 8 years, 1 of disease progression. In a pooled retrospective analysis of the French and United States cohorts in the rituximab era, with a slighter longer follow-up (10 years), ∼20% of patients died, primarily from disease progression.29 With all of the limitations dictated by a comparison between a real-world population and a clinical trial population, the survival rates observed with the use of R2 seem promising.
We acknowledge the limitations of our study, including its reporting from a single-center institution experience, and the retrospective interpretation of response to treatment of a fraction of patients due to a change in response criteria over the years. It is also important to note that indication of therapy was determined in this study by the treating physician, about half of the patients included in the analysis had high tumor burden disease, as defined by Groupe d'Etude des Lymphomes Folliculaires, and this did not affect outcome.
In summary, after extended follow-up, R2 remains an effective and safe frontline treatment for patients with FL. The regimen’s efficacy is independent of traditional prognostic factors, and studies aimed at improving the depth of response associated with its use may result in a lower rate of early progression and subsequent better long-term outcomes for these patients.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported, in part, by Anderson Cancer Center Support Grant P30 CA016672. P.S. is partly supported by the Lymphoma Research Foundation Career Development Award.
Authorship
Contribution: P.S. and P.J. analyzed data and wrote the manuscript; F.S., M.A.R., L.E.F., F.H., J.W., M.W., and S.S.N. provided clinical care to patients and coauthored the paper; R.J.J. and S.F. collected clinical data and coauthored the paper; L.F. provided statistical support and coauthored the paper; and N.H.F. and L.J.N. designed the study, analyzed the data, provided clinical care to patients, and wrote the manuscript.
Conflict-of-interest disclosure: S.S.N. reports honoraria and research support from Kite, a Gilead Company, Merck, and Celgene; research support from Bristol Myers Squibb, Poseida Therapeutics, Cellectis, Karus Therapeutics, and Acerta Pharma; and honoraria from Novartis, Pfizer, and Unum Therapeutics. F.S. reports honoraria from Celgene. L.J.N. reports honoraria from Celgene, Genentech, Gilead, Janssen Pharmaceuticals, Juno, Novartis, Spectrum, and TG Therapeutics and research support from Celgene, Genentech, Janssen Pharmaceuticals, Karus Therapeutics, and Merck. N.H.F. has served as a consultant for and received research funding from AbbVie, Janssen Pharmaceuticals, Celgene, and TG Therapeutics; has received research funding from Roche; and has had travel, accommodations, and expenses paid by Celgene and Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Nathan H. Fowler, Division of Cancer Medicine, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: nfowler@mdanderson.org; and Loretta J. Nastoupil, Division of Cancer Medicine, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: lnastoupil@mdanderson.org.
REFERENCES
Author notes
P.S. and P.J. contributed equally to this work.
L.J.N. and N.H.F. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal