Key points
Ulocuplumab with ibrutinib is tolerated in patients with CXCR4Mut WM with thrombocytopenia as the most frequent adverse event.
The combination was associated with short time to a major response, major responses
in all patients, and 90% 2-year progression-free survival.
Abstract
MYD88 and CXCR4 mutations are common in Waldenström macroglobulinemia (WM). Mutated CXCR4 (CXCR4Mut) impacts BTK-inhibitor response. We conducted a phase 1 trial of the CXCR4-antagonist ulocuplumab with ibrutinib in this first-ever study to target CXCR4Mut in WM. Ibrutinib was initiated at 420 mg/d with cycle 1 and continued until intolerance or progression; ulocuplumab was given cycles 1 to 6, with a 3 + 3 dose-escalation design. Each cycle was 4 weeks. Thirteen symptomatic patients, of whom 9 were treatment-naive patients were enrolled. Twelve were evaluable for response. At best response, their median serum immunoglobulin M declined from 5574 to 1114 mg/dL; bone marrow disease decreased from 65% to 10%, and hemoglobin increased from 10.1 to 14.2 g/dL (P < .001). The major and VGPR response rates were 100% and 33%, respectively, with VGPRs observed at lower ulocuplumab dose cohorts. Median times to minor and major responses were 0.9 and 1.2 months, respectively. With a median follow-up of 22.4 months, the estimated 2-year progression-free survival was 90%. The most frequent recurring grade ≥2 adverse events included reversible thrombocytopenia, rash, and skin infections. Ulocuplumab dose-escalation did not impact adverse events. The study demonstrates the feasibility of combining a CXCR4-antagonist with ibrutinib and provides support for the development of CXCR4-antagonists for CXCR4Mut WM. This trial was registered at www.clinicaltrials.gov as #NCT03225716.
Introduction
MYD88 and CXCR4 mutations are found in 95% to 97% and 30% to 40% of patients with Waldenström macroglobulinemia (WM), respectively.1-5 Mutated MYD88 (MYD88Mut) triggers BTK-related prosurvival signaling, whereas mutated CXCR4 (CXCR4Mut) promotes drug resistance through AKT and ERK activation in response to its ligand CXCL12.5-9 More than 40 nonsense and frameshift CXCR4 variants have been identified in WM.2-5 Mutations in CXCR4 prevent receptor downregulation in response to CXCL12, thereby potentiating downstream signaling.6-8 CXCR4Mut impacts disease presentation . Nonsense variants associate with high serum immunoglobulin M (IgM) levels, symptomatic hyperviscosity, and earlier treatment initiation in WM.4,10 CXCR4Mut is associated with a delayed response, fewer major responses, and shorter progression-free survival (PFS) to BTK-inhibitors in WM.11-15 CXCR4 antagonists, including ulocuplumab, sensitize CXCR4Mut expressing WM cells to ibrutinib.7-9 Ulocuplumab is a first-in-class fully human IgG4 monoclonal antibody that binds to CXCR4 and blocks ligand engagement. Response to CXCL12 is abrogated by ulocuplumab at 50% effective concentration of ≤35 nmol/L in various CXCR4-expressing cell models, including B-lymphoma cells.16 Ulocuplumab was evaluated alone and in combination in acute myeloid leukemia and myeloma with no dose-limiting toxicity up to 10 mg/kg per dose.17,18 We therefore investigated ulocuplumab and ibrutinib in patients with CXCR4Mut WM.
Study design
The study (#NCT03225716) was approved by our institutional review board, and participants provided informed written consent. Symptomatic patients meeting consensus guidelines for WM diagnosis and treatment, with MYD88Mut and CXCR4Mut disease, and BTK-inhibitor naive were eligible.19,20 MYD88 and CXCR4 mutations status was determined as before using CD19-selected bone marrow (BM) mononuclear cells.3 Other study criteria can be found in the protocol (supplemental Appendix 1, available on the Blood Web site). Ibrutinib was initiated at 420 mg/d with cycle 1 and continued until intolerance or progression; dose reduction for toxicity attributed to either drug was permitted per protocol (supplemental Appendix 1). Because delayed responses with ibrutinib monotherapy occurred in patients with CXCR4Mut WM for 5 to 7 months, ulocuplumab was given with ibrutinib during cycles 1 to 6.11,12 Each cycle was 4 weeks. For the first cycle, ulocuplumab was administered at 400 mg (cohort I) and 800 mg (cohorts II, III) IV once weekly; then 800 mg (cohort I), 1200 mg (cohort II), 1600 mg (cohort III) every other week during cycles 2 to 6. A 3 + 3 design was used for the phase 1 study. Dose-limiting toxicities are defined in section 5.4 of the protocol (supplemental Appendix 1). Responses were assessed using modified criteria from the 6th International Workshop on WM.11 PFS was estimated by Kaplan-Meier method. Pairwise comparisons were made using Wilcoxon signed rank test. Fisher’s exact probability test was used for categorical response comparisons. Cochran-Mantel-Haenszel test was used for analysis of matched categorical data. Values of P ≤ .05 were considered statistically significant. Deidentified collected participant data will be shared upon request.
Results and discussion
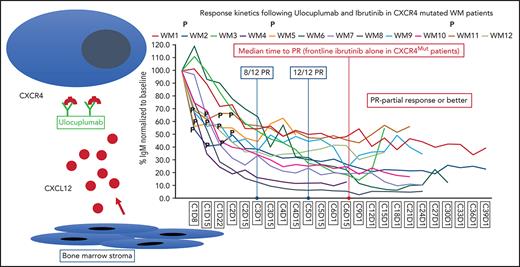
This is the first study to target CXCR4Mut in WM. CXCR4 is among the top differentially expressed genes in WM lymphoplasmacytic cells compared with healthy donor B cells, regardless of CXCR4 mutation status.21 Given the relatively high activity level of ibrutinib, including attainment of deeper and faster major responses among patients with CXCR4WT WM, this study focused on patients with CXCR4Mut WM. Thirteen patients, 9 previously untreated, were enrolled in the phase 1 study. Their median baseline characteristics were as follows: age 61.5 years (range, 40-76 years); serum IgM 5241 mg/dL (range, 2660-8215 mg/dL); BM disease involvement 60% (range, 20% to 95%); hemoglobin 9.1 g/dL (range, 8-13.1 g/dL); prior therapies 0 (range, 0-2). Five patients (42%) had symptomatic hyperviscosity as a treatment indication, a more common finding in patients with CXCR4Mut WM.10 All expressed MYD88L265P. CXCR4Mut included nonsense (n = 7) and frameshift (n = 6) variants. One patient on cohort I withdrew consent and was replaced after 1 dose of ulocuplumab per protocol. Twelve patients were therefore evaluable for response. At best response, their median serum IgM declined from 5574 mg/dL (range, 2660-8215 mg/dL) to 1114 mg/dL (range, 209-2914 mg/dL). Pretherapy, 10 of 12 patients (83.3%) had a serum IgM > 4000 mg/dL; following treatment, none had a serum IgM > 4000 mg/dL (P < .001). BM disease burden decreased from 65% (range, 20% to 95%) to 10% (range, 2% to 25%) (P < .001), whereas hemoglobin increased from 10.1 g/dL (range, 8-13.6 g/dL) to 14.2 g/dL (range, 13.1-17 g/dL) (P < .001). The overall and major (≥ partial response [PR]) response rate was 100%, and 4 of 12 (33%) patients attained a VGPR (2 with nonsense; 2 with frameshift variants). VGPRs were observed in cohorts I (n = 2) and II (n = 2), with similar median follow-up across all 3 cohorts (cohort I, 19.4 months; cohort II, 22.1 months; cohort III, 19.3 months).
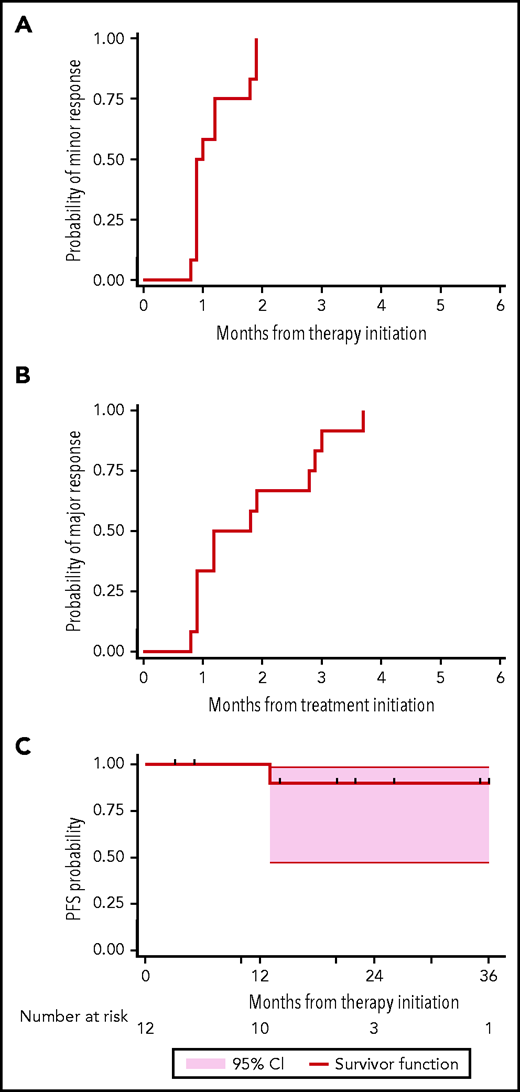
The time to minor and major responses are shown in Figure 1. The median time to a minor and a major response was 0.9 (95% confidence interval [CI], 0.9-1.8) and 1.2 months (95% CI, 0.9-2.8), respectively. Serial serum IgM levels for individual patients by dosing cohort is shown in supplemental Figure 1, with similar response kinetics observed across all ulocuplumab dosing cohorts. Eight (75%) and 12 (100%) of the 12 evaluable patients were major responders by cycles 3 and 5, respectively. Symptomatic hyperviscosity resolved in all 5 patients, and pheresis required in 3 ended by cycle 3. These data compare favorably to experiences with ibrutinib alone, wherein the median time to a major response is 4.7 to 7.3 months, and PR and VGPR attainment occurred in 60% to 70% and 9% to 14% of patients with CXCR4Mut WM, respectively.11,12,14 Moreover, these data compare favorably to combined ibrutinib and rituximab wherein time to major response was 3 months, and fewer (19%) VGPRs were attained in patients with CXCR4Mut WM with similar follow-up.22 The accelerated time to major response and attainment of deeper responses are important for many patients with WM whose morbidity is driven by the IgM paraprotein.5,23 With a median follow-up of 22.4 months (95% CI, 5.4-25.9 months), the estimated 2-year PFS was 90% (95% CI, 47% to 99%) (Figure 1 ). By comparison, 2-year estimated PFS with ibrutinib monotherapy was 30% to 70% in previously treated, and not available in treatment-naive patients with CXCR4Mut WM.11,12,14
Response and progression curves for 12 evaluable patients with WM following ulocuplumab and ibrutinib. Time to minor (A), major (B), and progression (C) are depicted. All patients were alive at end of study follow-up.
Response and progression curves for 12 evaluable patients with WM following ulocuplumab and ibrutinib. Time to minor (A), major (B), and progression (C) are depicted. All patients were alive at end of study follow-up.
Grade 2 to 4 toxicities at least possibly related to protocol therapy are listed in Table 1 for all 13 patients, including the patient replaced after 1 dose of ulocuplumab. This patient had grade 1 treatment-related thrombocytopenia and grade 1 hematuria related to a preexisting renal cyst. The most frequent, recurring grade ≥2 adverse events included thrombocytopenia (n = 8), rashes (n = 5), skin infections (n = 4), fatigue (n = 4), diarrhea (n = 3), cough (n = 3), and hyperglycemia (n = 3). Dose intensity of ulocuplumab did not impact adverse events (supplemental Table 2). Two patients (one for persistent fatigue, one for rash) were ibrutinib dose-reduced to 280 mg/d. Ulocuplumab infusions were well tolerated, with no notable infusion-related toxicities. Eleven of 12 evaluable patients received all 6 cycles; 1 patient received 4 cycles. Toxicities were reversible with dose-modification or discontinuation. Decreases in serum IgA and IgG occurred, akin to our previous trial of untreated WM patients with ibrutinib alone.12 Median IgA levels declined from 36.5 mg/dL (range, 5-146 mg/dL) to 19 mg/dL (range, 5-63 mg/dL; P = .017), whereas IgG levels declined from 391 mg/dL (range, 131-632 mg/dL) to 235.5 mg/dL (range, 97-495 mg/dL; P = .002).
Adverse events associated with ulocuplumab and ibrutinib therapy in 13 patients with WM
| Adverse event . | Grade 2 . | Grade 3 . | Grade 4 . | Total grades 2 to 4 . |
|---|---|---|---|---|
| Blood and lymphatic system disorders | ||||
| Anemia | 1 | 0 | 0 | 1 |
| Thrombocytopenia | 4 | 3 | 1 | 8 |
| Neutropenia | 0 | 0 | 1 | 1 |
| Cardiac disorders | ||||
| Atrial fibrillation | 1 | 0 | 0 | 1 |
| Supraventricular tachycardia | 0 | 1 | 0 | 1 |
| Palpitations | 1 | 0 | 0 | 1 |
| Constitutional | ||||
| Chills | 1 | 0 | 0 | 1 |
| Fatigue | 4 | 0 | 0 | 4 |
| Gastrointestinal disorders | ||||
| Diarrhea | 3 | 0 | 0 | 3 |
| Gastric ulcer/gastritis | 1 | 0 | 0 | 1 |
| Gastroesophageal reflux disease | 1 | 0 | 0 | 1 |
| Mucositis oral | 1 | 0 | 0 | 1 |
| Other | 1 | 0 | 0 | 1 |
| General disorders | ||||
| Edema limbs | 1 | 0 | 0 | 1 |
| Infections and infestations | ||||
| Ear infection/otitis media | 2 | 0 | 0 | 2 |
| Eye infection | 1 | 0 | 0 | 1 |
| Lung infection | 2 | 0 | 0 | 2 |
| Dental infection | 1 | 0 | 0 | 1 |
| Sinusitis | 2 | 0 | 0 | 2 |
| Skin infection | 4 | 0 | 0 | 4 |
| Upper respiratory infection | 2 | 0 | 0 | 2 |
| Urinary tract infection | 1 | 0 | 0 | 1 |
| Oral candidiasis | 1 | 0 | 0 | 1 |
| Metabolism and nutrition disorders | ||||
| Hyperglycemia | 1 | 2 | 0 | 3 |
| Hypokalemia | 0 | 1 | 0 | 1 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 3 | 0 | 0 | 3 |
| Myalgia | 2 | 0 | 0 | 2 |
| Lightheadedness | 1 | 0 | 0 | 1 |
| Headache | 0 | 1 | 0 | 1 |
| Presyncope | 1 | 0 | 0 | 1 |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough | 3 | 0 | 0 | 3 |
| Dyspnea | 2 | 1 | 0 | 3 |
| Hypoxia | 1 | 0 | 0 | 1 |
| Epistaxis | 1 | 0 | 0 | 1 |
| Other | 2 | 0 | 0 | 2 |
| Skin and subcutaneous tissue disorders | ||||
| Skin and subcutaneous tissue disorders, rash | 5 | 1 | 0 | 6 |
| Skin and subcutaneous tissue disorders, other | 1 | 0 | 0 | 1 |
| Adverse event . | Grade 2 . | Grade 3 . | Grade 4 . | Total grades 2 to 4 . |
|---|---|---|---|---|
| Blood and lymphatic system disorders | ||||
| Anemia | 1 | 0 | 0 | 1 |
| Thrombocytopenia | 4 | 3 | 1 | 8 |
| Neutropenia | 0 | 0 | 1 | 1 |
| Cardiac disorders | ||||
| Atrial fibrillation | 1 | 0 | 0 | 1 |
| Supraventricular tachycardia | 0 | 1 | 0 | 1 |
| Palpitations | 1 | 0 | 0 | 1 |
| Constitutional | ||||
| Chills | 1 | 0 | 0 | 1 |
| Fatigue | 4 | 0 | 0 | 4 |
| Gastrointestinal disorders | ||||
| Diarrhea | 3 | 0 | 0 | 3 |
| Gastric ulcer/gastritis | 1 | 0 | 0 | 1 |
| Gastroesophageal reflux disease | 1 | 0 | 0 | 1 |
| Mucositis oral | 1 | 0 | 0 | 1 |
| Other | 1 | 0 | 0 | 1 |
| General disorders | ||||
| Edema limbs | 1 | 0 | 0 | 1 |
| Infections and infestations | ||||
| Ear infection/otitis media | 2 | 0 | 0 | 2 |
| Eye infection | 1 | 0 | 0 | 1 |
| Lung infection | 2 | 0 | 0 | 2 |
| Dental infection | 1 | 0 | 0 | 1 |
| Sinusitis | 2 | 0 | 0 | 2 |
| Skin infection | 4 | 0 | 0 | 4 |
| Upper respiratory infection | 2 | 0 | 0 | 2 |
| Urinary tract infection | 1 | 0 | 0 | 1 |
| Oral candidiasis | 1 | 0 | 0 | 1 |
| Metabolism and nutrition disorders | ||||
| Hyperglycemia | 1 | 2 | 0 | 3 |
| Hypokalemia | 0 | 1 | 0 | 1 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 3 | 0 | 0 | 3 |
| Myalgia | 2 | 0 | 0 | 2 |
| Lightheadedness | 1 | 0 | 0 | 1 |
| Headache | 0 | 1 | 0 | 1 |
| Presyncope | 1 | 0 | 0 | 1 |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough | 3 | 0 | 0 | 3 |
| Dyspnea | 2 | 1 | 0 | 3 |
| Hypoxia | 1 | 0 | 0 | 1 |
| Epistaxis | 1 | 0 | 0 | 1 |
| Other | 2 | 0 | 0 | 2 |
| Skin and subcutaneous tissue disorders | ||||
| Skin and subcutaneous tissue disorders, rash | 5 | 1 | 0 | 6 |
| Skin and subcutaneous tissue disorders, other | 1 | 0 | 0 | 1 |
Grade ≥2 adverse events deemed by investigators to be possibly, probably, or definitely associated with protocol therapy are shown. Number of individual patients with the indicated toxicity is shown, with the highest toxicity grade shown for an individual patient. In addition, 4 patients had grade 1 thrombocytopenia.
Three of 12 evaluable patients came off protocol therapy; 1 previously treated with bendamustine and rituximab, who transformed to diffuse large B-cell lymphoma and received R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine (oncovin), and prednisone); and 2 for toxicity: one after 4 cycles with new headaches, and small hemorrhagic appearing central nervous system lesions with grade 1 thrombocytopenia that resolved off therapy and was at least possibly related to ibrutinib or ulocuplumab; and another after 6 cycles for a grade 3 maculopapular trunk and extremity rash with pathology consistent with a hypersensitivity reaction that resolved off treatment and prolonged steroid course. Although hypersensitivity-related rashes have not been reported with ulocuplumab, such reactions are common with ibrutinib and were likely etiological for this patient’s rash.24
In summary, the combination of ulocuplumab and ibrutinib led to a short time to major response, attainment of major responses in all patients, and an estimated 2-year PFS of 90%, findings which favorably compare against ibrutinib alone in this patient population. No unexpected toxicities were observed, and our findings showed that ulocuplumab at the highest dose cohort examined in this study was well tolerated and did not accentuate adverse events. Although the results support further investigation of ulocuplumab and ibrutinib in CXCR4Mut WM, sponsor termination of ulocuplumab development for lack of meaningful activity in other diseases prevented a planned phase 2 study. Our findings suggest that ibrutinib with ulocuplumab at dosing administered in cohort III could be considered for such a trial. The findings from these studies may be relevant to other therapeutics impacted by CXCR4Mut and support the development of other CXCR4 antagonists.23,25 A phase 1 study examining mavorixafor, an oral antagonist of CXCR4 along with ibrutinib, was recently initiated (#NCT04274738) in CXCR4Mut WM. In addition, because CXCR4 is overexpressed in CXCR4WT WM and may be both biologically and therapeutically relevant, future studies with CXCR4 antagonists can be considered in this population.7-9,22 In conclusion, our findings demonstrate the feasibility of combining a CXCR4-antagonist with ibrutinib and provides support for the development of CXCR4-antagonists for CXCR4Mut WM.
Acknowledgments
Bristol Myers Squibb supported this investigator-initiated study and provided research funding and ulocuplumab. Ibrutinib was commercially sourced. The authors acknowledge the generous support of Peter Bing, the Siegel Family Fund for WM, the David and Janet Bingham Research Fund of the International Waldenström’s Macroglobulinemia Foundation, the Yang Family Research Fund of the International Waldenström’s Macroglobulinemia Foundation, a Leukemia and Lymphoma Society Translational Research Grant R6507-18, and a National Institutes of Health, National Cancer Institute SPORE grant in multiple myeloma (2P50CA100707-16A1).
Authorship
Contribution: S.P.T., J.J.C., and K.M. designed the study; J.J.C., A.R.B., I.M.G., S.R.S., C.A.F., K.M., C.R.L., and T.P.W. provided treatment to study patients and/or collected study data; K.M., C.R.L., and T.P.W. coordinated the study, collected study data, and/or provided regulatory oversight; L.X., X.L., G.Y., C.J.P., M.M., A.K., M.L.G., M.G.D., N.T., and Z.R.H. processed tumor samples and performed MYD88 and CXCR4 genotyping; Y.C., G.Y., X.L., L.X., A.M.R., and A.S. performed translational studies in support of this study; S.P.T., J.J.C., K.M., and Z.R.H. performed data analysis; S.P.T. wrote the first draft of the manuscript; and all the authors approved the final version of the manuscript.
Conflict-of-interest disclosure: S.P.T., G.Y., A.R.B., Z.R.H., and J.J.C. have received research funding, honoraria, and/or consulting fees from Pharmacyclics Inc, Janssen Pharmaceuticals Inc, the manufacturer of ibrutinib, X4 Pharmaceuticals, and Beigene. S.P.T. has received research funding from Bristol Myers Squibb, X4 Pharmaceuticals, Eli Lilly, and Beigene. J.J.C. received research funding and/or consulting fees from Abbvie, Beigene, Roche, and TG Therapeutics. A.R.B. has received consulting fees from Beigene. I.M.G. has served on the advisory board of Janssen, BMS, GSK, Binding Site, Takeda, and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana Farber Cancer Institute, M548, 450 Brookline Ave, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal