Key Points
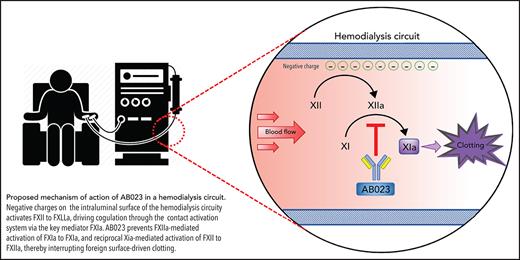
The FXI antibody AB023 inhibits contact activation by binding FXI and blocking its activation by activated factor XII.
AB023 reduced dialyzer clotting and thrombin-antithrombin complexes compared with placebo during heparin-free dialysis in a phase 2 trial.
Abstract
End-stage renal disease (ESRD) patients on chronic hemodialysis have repeated blood exposure to artificial surfaces that can trigger clot formation within the hemodialysis circuit. Dialyzer clotting can lead to anemia despite erythropoietin and iron supplementation. Unfractionated heparin prevents clotting during hemodialysis, but it is not tolerated by all patients. Although heparin-free dialysis is performed, intradialytic blood entrapment can be problematic. To address this issue, we performed a randomized, double-blind, phase 2 study comparing AB023, a unique antibody that binds factor XI (FXI) and blocks its activation by activated FXII, but not by thrombin, to placebo in 24 patients with ESRD undergoing heparin-free hemodialysis. Patients were randomized to receive a single predialysis dose of AB023 (0.25 or 0.5 mg/kg) or placebo in a 2:1 ratio, and safety and preliminary efficacy were compared with placebo and observations made prior to dosing within each treatment arm. AB023 administration was not associated with impaired hemostasis or other drug-related adverse events. Occlusive events requiring hemodialysis circuit exchange were less frequent and levels of thrombin-antithrombin complexes and C-reactive protein were lower after AB023 administration compared with data collected prior to dosing. AB023 also reduced potassium and iron entrapment in the dialyzers, consistent with less blood accumulation within the dialyzers. We conclude that despite the small sample size, inhibition of contact activation–induced coagulation with AB023 was well tolerated and reduced clotting within the dialyzer. This trial was registered at www.clinicaltrials.gov as #NCT03612856.
Introduction
Development of the hemodialysis apparatus >75 years ago and subsequent improvements in disposable biomaterials that reduce thrombogenicity have significantly extended life expectancy for patients with end-stage renal disease (ESRD). However, even with the newest hemodialyzers, most procedures require systemic anticoagulation to reduce clot formation in the extracorporeal circuit and prevent chronic blood loss that contributes to the anemia of ESRD patients.1 The anticoagulant of choice remains unfractionated heparin (UFH) due to its low cost, short biological half-life, and convenience of use. UFH administered at the beginning of dialysis reduces circuit clotting but increases the risk of access site bleeding until it is cleared from the circulation.2 In addition, heparin-induced thrombocytopenia is a potentially severe complication that requires alternative anticoagulation approaches.3-6 Nonheparin anticoagulants, heparin-free dialysis, and heparin-bonded circuitry have been explored as alternatives to IV heparin. However, complications such as bleeding and circuit clotting remain unsolved issues.7,8
Our group and others have shown that contact activation contributes to blood clotting and red blood cell entrapment in blood-perfused prosthetic vascular grafts and extracorporeal oxygenators.9,10 Generation of activated coagulation factor XII (FXIIa) can produce, among others, thrombin and bradykinin. Blockade or knockout of FXII, FXI, or FXIIa-mediated activation of FXI is antithrombotic and anti-inflammatory in various animal models.9-12 While FXI deficiency results in a mild/moderate bleeding disorder, FXII deficiency is asymptomatic.13,14 Thus, inhibiting contact activation of FXI may reduce device-associated clotting without the bleeding risks of traditional anticoagulants.
AB023 (xisomab 3G3) is a novel antithrombotic recombinant antibody that binds to and reduces FXI activation by FXIIa as well as reciprocal activation of FXII by FXIa.11 AB023 is different from other FXI inhibitors, since it preserves hemostatic FXI activation by thrombin and FXIa activation of FIX. AB023 demonstrated a favorable safety profile in a phase 1 clinical trial.15 In that trial, AB023 at 0.5 mg/kg prolonged the activated partial thromboplastin time (aPTT) of healthy volunteers for 1 week. Given the compelling rationale for inhibiting contact activation of FXI, we performed a phase 2 clinical trial in ESRD patients to assess the safety and clot-reducing efficacy of AB023 compared with placebo during heparin-free hemodialysis.
Methods
The study is registered at www.clinicaltrials.gov as #NCT03612856.
Study design and oversight
This phase 2, randomized, double-blind, placebo-controlled, single-dose trial to determine the safety and preliminary efficacy of AB023 in patients with ESRD on chronic hemodialysis was performed at the Orlando Clinical Research Center (Orlando, FL). The full protocol is available in supplemental Methods (available on the Blood Web site). The study was approved by the IntegReview Institutional Review Board before initiation as required by and conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals Good Clinical Practice. Written informed consent was obtained from all participants before trial enrollment. A safety data committee performed safety data reviews throughout the trial. All authors had access to study data.
Participants
Patients 18 to 80 years of age with ESRD on a stable, 3-times-per-week outpatient hemodialysis regimen for >3 months utilizing an arteriovenous (AV) fistula or AV graft were eligible for study enrollment. Patients were excluded if dialysis access was via a catheter or if they were on continuous anticoagulation and/or antiplatelet therapy or had a history of venous or arterial thromboembolic events within the preceding 3 months, a platelet count <75 x 109/L, an international normalized ratio >1.4, or an aPTT >1.5 times the upper limit of normal. The full list of inclusion/exclusion criteria is provided in supplemental Methods. Patients were recruited from a site database as well as through advertisements.
Randomization and study treatment
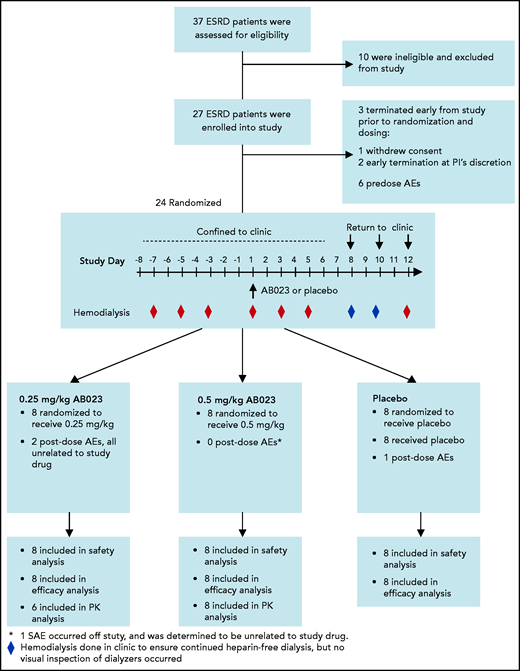
Before study drug administration, all subjects underwent 3 heparin-free hemodialysis sessions to obtain baseline data. Patients were enrolled sequentially into AB023 dosing cohorts 1 and 2. Within each cohort, patients were randomly assigned to receive a single bolus of AB023 or placebo in a 2:1 ratio. AB023 or placebo was injected into the dialysis line proximal to the dialyzer at the start of hemodialysis on study day 1. Patients in cohort 1 received 0.25 mg/kg AB023. Once target enrollment was reached in cohort 1, patients were assigned to cohort 2, in which 0.5 mg/kg-AB023 or placebo was given. The study timeline and procedures are illustrated in Figure 1. Additional details are outlined in the protocol.
Enrollment, randomization, populations for analysis, and study timeline in the AB023 phase 2 clinical trial. Thirty-seven patients were screened for study eligibility. Ten patients did not meet inclusion/exclusion criteria and were not enrolled in the study. An additional 3 patients terminated the study early, prior to randomization on study day 1. Twenty-four patients were randomized and all 24 patients completed the study. All randomized patients were confined to the clinic from study day −8 to study day 6, after which they returned to the clinic on study days 8, 10, and 12 to continue heparin-free hemodialysis. Predose hemodialysis occurred on study days −7, −5, and −3. Dosing occurred on study day 1, and postdosing hemodialysis occurred on study days 1, 3, 5, 8, 10, and 12. The red diamonds represent hemodialysis days in which data were collected, and the blue diamonds represent heparin-free hemodialysis sessions that were performed in the clinic, but no data were collected. Cohorts were dosed sequentially, starting with cohort 1 (0.25 mg/kg or matching placebo). Once cohort 1 was completed, dosing for cohort 2 began (0.5 mg/kg or matching placebo). Placebo from both cohorts were pooled together for analysis. All 24 patients randomized were included in the safety and efficacy analyses. Two patients from the 0.25 mg/kg group were excluded from the PK analysis; 1 patient was excluded because the percent of AUC0-inf extrapolated (AUC%extrap) was very high (63%) and therefore did not meet the prespecified criteria for inclusion of the PK analyses, and 1 patient was excluded from the summary statistics because the 0.17-hour AB023 concentration was an outlier. AEs, adverse events; SAE, serious adverse event.
Enrollment, randomization, populations for analysis, and study timeline in the AB023 phase 2 clinical trial. Thirty-seven patients were screened for study eligibility. Ten patients did not meet inclusion/exclusion criteria and were not enrolled in the study. An additional 3 patients terminated the study early, prior to randomization on study day 1. Twenty-four patients were randomized and all 24 patients completed the study. All randomized patients were confined to the clinic from study day −8 to study day 6, after which they returned to the clinic on study days 8, 10, and 12 to continue heparin-free hemodialysis. Predose hemodialysis occurred on study days −7, −5, and −3. Dosing occurred on study day 1, and postdosing hemodialysis occurred on study days 1, 3, 5, 8, 10, and 12. The red diamonds represent hemodialysis days in which data were collected, and the blue diamonds represent heparin-free hemodialysis sessions that were performed in the clinic, but no data were collected. Cohorts were dosed sequentially, starting with cohort 1 (0.25 mg/kg or matching placebo). Once cohort 1 was completed, dosing for cohort 2 began (0.5 mg/kg or matching placebo). Placebo from both cohorts were pooled together for analysis. All 24 patients randomized were included in the safety and efficacy analyses. Two patients from the 0.25 mg/kg group were excluded from the PK analysis; 1 patient was excluded because the percent of AUC0-inf extrapolated (AUC%extrap) was very high (63%) and therefore did not meet the prespecified criteria for inclusion of the PK analyses, and 1 patient was excluded from the summary statistics because the 0.17-hour AB023 concentration was an outlier. AEs, adverse events; SAE, serious adverse event.
At enrollment, patients were confined to the clinical research unit on study day −8 through study day 6. Patients then returned to the clinic on study days 8, 10, and 12 to ensure heparin-free hemodialysis for the remainder of the trial. From study day −7 (1 week prior to dosing on day 1) through day −1, all patients underwent heparin-free hemodialysis 3 times (days −7, −5, and −3). On study day 1, patients received either AB023 or placebo and then underwent hemodialysis on study days 1, 3, and 5. On study day 6, patients were released from the clinic and returned on study days 8, 10, and 12 for heparin-free hemodialysis. Each hemodialysis session lasted 4 hours, and each patient was assessed for all scheduled procedures and end points before, during, and after each hemodialysis session.
Dialysis was performed using Fresenius Optiflux F180NR dialyzers, and all groups underwent heparin-free hemodialysis for the duration of the trial.16 After trial completion, patients returned to their routine hemodialysis regimens.
Study outcomes
Safety
The primary safety outcome was the number and severity of adverse events, including hemostatic safety (time to achieve hemostasis) at the vascular access site after each hemodialysis session. After hemodialysis, the access needles were removed from the vascular access sites and a pressure dressing was applied for 10 minutes. The site was then assessed, and if bleeding persisted, the pressure dressing was reapplied and the site was assessed every 5 minutes until hemostasis was achieved.
Efficacy
Outcomes included visual scoring of clots in the dialyzer and venous chambers, recording of the number and volume of saline flushes required to maintain circuit patency, and noting of thrombotic circuit occlusions requiring circuit replacement. Following each hemodialysis session, the dialyzer cartridge, tubing, and venous chamber were flushed with saline to return blood to the patient. Thrombus accumulation in the visible areas of the transparent dialyzer (blood loss by entrapment due to clotting) was assessed by visual inspection of the dialyzer membrane and venous chamber, and a score was assigned using a standardized visual assessment scale (supplemental Table 1) by assessors who were blinded as to treatment allocation.7,17-20 If complete occlusion of the hemodialysis circuit occurred, site standard operating procedures were followed to replace the clotted extracorporeal circuit and resume hemodialysis, and the event was noted. Following visual assessment of the dialyzers on site, the cartridges were stored frozen until further analyses took place. To assess the extent of clotting inside the dialyzers, cartridges were thawed, emptied of saline, and filled with distilled water for 1 week. The water was then collected from the dialyzer, and its potassium and iron concentrations were determined as an index of red blood cell accumulation using inductively coupled plasma mass spectrometry (ICP-MS) in kinetic energy discrimination mode as described elsewhere.21 All dialyzers from a single subject were processed together, and the average of the predose sessions for each subject was calculated and compared with those for day 1 postdosing with drug or placebo. For subjects requiring a circuit change during a hemodialysis session, potassium and iron concentration results for each dialyzer were combined. Method development experiments where defined volumes of human blood were clotted in primed hemodialyzers demonstrated a linear relationship between the clot volume inside the hollow fibers and the potassium concentrations in water from the dialysate chamber as described above (supplemental Figure 1).
Exploratory end points
Exploratory efficacy end points included systemic thrombin-antithrombin (TAT) complex levels measured in plasma samples taken on study day 1 predose and 4 hours postdose using enzyme-linked immunosorbent assay (ELISA) kits (TAT-micro, Enzygnost, Siemens). To assess the effect of AB023 on inflammatory response of a single hemodialysis session, plasma levels of C-reactive protein (CRP) and interleukin-6 (IL-6) were measured in samples taken predose on study day 1 and 12, 24, and 48 hours postdose using ELISA kits (R&D Systems).
Hemodialytic efficacy assessments
The efficiency of each hemodialysis session was evaluated by measurements of blood urea nitrogen (BUN) and potassium levels before and after dialysis. The fractional clearance of urea (Kt/V) and urea reduction ratio (URR), metrics endorsed by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative, were calculated.22
PK and PD
Blood samples for pharmacokinetic (PK) and pharmacodynamic (PD) assessment were collected on study day 1 predose and at 0.167, 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 48, 96, 120 and 264 hours after AB023 or placebo administration. Additional blood samples at 168 and 216 hours after dosing were collected for PD assessment only. Free plasma AB023 concentrations were determined using a validated ELISA (Celerion, Lincoln, NE). PK measurements included total exposure, exposure up to last measurable concentration, elimination rate constant, maximum plasma concentration, clearance, half-life of elimination, mean absorption time, and volume of distribution at steady state. aPTT was measured at a single central laboratory (Eccolabs, Tampa, FL) and used as a PD biomarker. Ratios were established by dividing assay results by the baseline aPTT (defined as the aPTT on day 1 before dosing). Development of anti-AB023 antibodies (anti-drug antibodies) was evaluated in patient samples with a validated ELISA using AB023 to capture anti-drug antibodies and biotinylated AB023 and streptavidin-horseradish peroxidase for detection.
Statistical analyses
Data analysis was performed by Aronora, as well as the contract research organization Celerion, which assisted in trial management. All patients who underwent randomization were included in the intention-to-treat analysis. The safety assessment included all patients who underwent randomization and received study drug and included events that occurred from day −8 at check-in to the clinic through the end of the study on day 12. Data from subjects who received placebo were pooled across cohorts.
The efficacy analysis was exploratory and thus unpowered. The study was designed to evaluate effects of AB023 by comparing the predose hemodialysis sessions (days −7, −5, and −3) to postdose hemodialysis sessions (days 1, 3, and 5) within individual cohorts. A comparison was also made across cohorts where appropriate.
Data were collected for 3 predose (days −7, −5, and −3) and 4 postdose days (days 1, 3, 5, and 12) for 168 individual hemodialysis sessions. The frequency of high-grade dialyzer clotting (score of ≥3 for blood entrapment in the dialyzer)18 was calculated for each group predose and compared with the frequency of severe clotting events postdose for each treatment group. Total events were calculated to generate percentage values. The number of times a score occurred in a treatment group (either from dialyzer or venous chamber) during the evaluation time was calculated as a percentage. For example, each treatment group has 8 subjects, and each subject underwent 3 predose hemodialysis sessions, so 8 × 3 = 24 total hemodialysis events were evaluated. If during this predose time a score of 3 occurred 6 times, the frequency of a score of 3 would be 6/24 = 0.25 × 100 = 25%.
Analysis of PD and biomarker data included data from day −7 through day 12 for all 24 randomized subjects. Summary statistics, including number of subjects (n), mean, standard deviation (SD), coefficent of variation (CV%), standard error of the mean (SEM), minimum, median, and maximum were calculated for all nominal values time points. Differences between comparable means were considered statistically significant at P < .05.
PK analysis was performed for all 16 patients that received study drug, except for 2 subjects in the 0.25 mg/kg group. Data from 1 subject in cohort 1 were excluded from analyses because the data did not meet the prespecified criteria for inclusion, and data from a second subject in cohort 1 were excluded because the AB023 concentration measured in the first sample was 16-fold higher than the amount administered, likely because of incomplete flushing of the line after antibody administration. Appropriate noncompartmental PK parameters were calculated from plasma-free AB023 concentration–time data using Phoenix WinNonlin version 8.1. PK concentrations and/or PK parameter descriptive statistics were generated using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patients
Twenty-seven subjects with ESRD were enrolled in the study. Three subjects attended study check-in on day −8 but were not randomized; 2 subjects at the discretion of the principal investigator (PI; 1 because of concerns about vascular access and 1 because they presented with nausea, vomiting, and diarrhea), and 1 subject withdrew consent. Twenty-four subjects with ESRD (19 males and 5 females) underwent randomization, and all completed the study. Baseline characteristics were similar in the 3 study groups. A summary of subject disposition can be found in Table 1. Analysis populations are shown in Figure 1.
Patient demographics
| . | Randomized patients . | ||
|---|---|---|---|
| 0.25 mg/kg AB023 (n = 8) . | 0.5 mg/kg AB023 (n = 8) . | Placebo (n = 8) . | |
| Age, mean (SD), y | 55.8 (7.6) | 53.4 (5.0) | 52.5 (9.2) |
| Women, n (%) | 1 (12.5) | 3 (37.5) | 1 (12.5) |
| Men, n (%) | 7 (87.5) | 5 (62.5) | 7 (87.5) |
| Race | |||
| White | 0 (0) | 1 (12.5) | 2 (25) |
| Black | 8 (100) | 7 (87.5) | 6 (75) |
| Weight, mean (SD), kg | 88.3 (17.1) | 82.9 (19.4) | 78.8 (10.4) |
| BMI, mean (SD) | 27.8 (3.9) | 27.9 (5.4) | 24.7 (2.9) |
| Smoker, n (%) | 1 (12.5) | 2 (25) | 1 (12.5) |
| Time on hemodialysis, mean (SD), y | 9.6 (6.7) | 7.6 (4.9) | 8 (5.3) |
| Primary cause of kidney failure, n (%) | |||
| Hypertension | 6 (75) | 5 (62.5) | 4 (50) |
| Diabetes | 0 | 2 (25) | 0 (0) |
| Polycystic kidney disease | 1 (12.5) | 0 (0) | 0 (0) |
| Multiple | 1 (12.5) | 1 (12.5) | 4 (50) |
| aPTT at screening, mean, (SD), s | 36.5 (3.6) | 39.3 (5.6) | 35.0 (4.1) |
| aPTT above normal range, n (%) | 3 (37.5) | 6 (75) | 2 (25) |
| Platelet count <150 000 at check-in, n (%) | 3 (37.5) | 3 (37.5) | 4 (50) |
| Platelet count at screening, mean (SD), K/µL | 220.1 (137.5) | 180.1 (72.3) | 164.4 (69.6) |
| Hemoglobin at screening, mean (SD), g/dL | 12.7 (1.9) | 12.1 (2.1) | 11.6 (0.6) |
| Blood pressure at screening, mean (SD), mm Hg | |||
| Systolic | 121 (27) | 135 (17) | 149 (30) |
| Diastolic | 76 (10) | 73 (12) | 83 (10) |
| Comorbidities, n (%) | |||
| Hypertension | 8 (100) | 8 (100) | 8 (100) |
| Diabetes | 1 (12.5) | 4 (50) | 5 (62.5) |
| Cardiovascular disease* | 2 (25) | 4 (50) | 2 (25) |
| History of extremity amputation | 1 (12.5) | 2 (25) | 0 (0) |
| Cancer | 0 (0) | 0 (0) | 1 (12.5) |
| . | Randomized patients . | ||
|---|---|---|---|
| 0.25 mg/kg AB023 (n = 8) . | 0.5 mg/kg AB023 (n = 8) . | Placebo (n = 8) . | |
| Age, mean (SD), y | 55.8 (7.6) | 53.4 (5.0) | 52.5 (9.2) |
| Women, n (%) | 1 (12.5) | 3 (37.5) | 1 (12.5) |
| Men, n (%) | 7 (87.5) | 5 (62.5) | 7 (87.5) |
| Race | |||
| White | 0 (0) | 1 (12.5) | 2 (25) |
| Black | 8 (100) | 7 (87.5) | 6 (75) |
| Weight, mean (SD), kg | 88.3 (17.1) | 82.9 (19.4) | 78.8 (10.4) |
| BMI, mean (SD) | 27.8 (3.9) | 27.9 (5.4) | 24.7 (2.9) |
| Smoker, n (%) | 1 (12.5) | 2 (25) | 1 (12.5) |
| Time on hemodialysis, mean (SD), y | 9.6 (6.7) | 7.6 (4.9) | 8 (5.3) |
| Primary cause of kidney failure, n (%) | |||
| Hypertension | 6 (75) | 5 (62.5) | 4 (50) |
| Diabetes | 0 | 2 (25) | 0 (0) |
| Polycystic kidney disease | 1 (12.5) | 0 (0) | 0 (0) |
| Multiple | 1 (12.5) | 1 (12.5) | 4 (50) |
| aPTT at screening, mean, (SD), s | 36.5 (3.6) | 39.3 (5.6) | 35.0 (4.1) |
| aPTT above normal range, n (%) | 3 (37.5) | 6 (75) | 2 (25) |
| Platelet count <150 000 at check-in, n (%) | 3 (37.5) | 3 (37.5) | 4 (50) |
| Platelet count at screening, mean (SD), K/µL | 220.1 (137.5) | 180.1 (72.3) | 164.4 (69.6) |
| Hemoglobin at screening, mean (SD), g/dL | 12.7 (1.9) | 12.1 (2.1) | 11.6 (0.6) |
| Blood pressure at screening, mean (SD), mm Hg | |||
| Systolic | 121 (27) | 135 (17) | 149 (30) |
| Diastolic | 76 (10) | 73 (12) | 83 (10) |
| Comorbidities, n (%) | |||
| Hypertension | 8 (100) | 8 (100) | 8 (100) |
| Diabetes | 1 (12.5) | 4 (50) | 5 (62.5) |
| Cardiovascular disease* | 2 (25) | 4 (50) | 2 (25) |
| History of extremity amputation | 1 (12.5) | 2 (25) | 0 (0) |
| Cancer | 0 (0) | 0 (0) | 1 (12.5) |
Includes cardiac surgery, stent placement, coronary artery disease, aneurism, congestive heart failure, stroke, and myocardial infarction.
Safety
Three of 24 (12.5%) subjects experienced a total of 3 grade 2 treatment-emergent adverse events (viral gastroenteritis), 2 of 16 (12.5%) following AB023 and 1 of 8 (12.5%) following placebo. None were deemed treatment related by the PI and the independent medical monitors of the study. These adverse events, both pre- and postdose, are shown in Table 2.
Adverse events for the AB023 phase 2 clinical trial
| Adverse event . | Severity . | Treatment group . | Onset . |
|---|---|---|---|
| Dialysis circuit hypotension | Grade 3 | 0.25 mg/kg | Predose |
| Viral gastroenteritis | Grade 2 | 0.25 mg/kg | Postdose |
| Viral gastroenteritis | Grade 2 | 0.25 mg/kg | Postdose |
| Viral gastroenteritis | Grade 2 | Placebo | Postdose |
| Vomiting | Grade 1 | 0.5 mg/kg | Predose |
| Vomiting | Grade 1 | N/A early term | Predose |
| Diarrhea | Grade 1 | 0.5 mg/kg | Predose |
| Diarrhea | Grade 1 | Placebo | Predose |
| Diarrhea | Grade 1 | N/A early term | Predose |
| Diarrhea | Grade 1 | Placebo | Predose |
| Nausea | Grade 1 | 0.5 mg/kg | Predose |
| Nausea | Grade 1 | N/A early term | Predose |
| Adverse event . | Severity . | Treatment group . | Onset . |
|---|---|---|---|
| Dialysis circuit hypotension | Grade 3 | 0.25 mg/kg | Predose |
| Viral gastroenteritis | Grade 2 | 0.25 mg/kg | Postdose |
| Viral gastroenteritis | Grade 2 | 0.25 mg/kg | Postdose |
| Viral gastroenteritis | Grade 2 | Placebo | Postdose |
| Vomiting | Grade 1 | 0.5 mg/kg | Predose |
| Vomiting | Grade 1 | N/A early term | Predose |
| Diarrhea | Grade 1 | 0.5 mg/kg | Predose |
| Diarrhea | Grade 1 | Placebo | Predose |
| Diarrhea | Grade 1 | N/A early term | Predose |
| Diarrhea | Grade 1 | Placebo | Predose |
| Nausea | Grade 1 | 0.5 mg/kg | Predose |
| Nausea | Grade 1 | N/A early term | Predose |
All adverse events were unrelated to the study drug.
N/A, not applicable; term, termination.
There was no clinically relevant bleeding events on study, and time to hemostasis at vascular access sites was unchanged after study drug administration compared with before (Table 3). One patient randomized to the 0.5 mg/kg dose arm of AB023 developed a subdural hematoma 32 days after dosing. This event occurred after a severe coughing episode. Based on timing (normal clotting profile [prothrombin time (PT) and aPTT] for >3 weeks), the subdural hematoma was deemed by the study PI and medical monitors to be unrelated to the study drug. No anti-drug antibodies were detected, and there were no deaths.
Time to hemostasis
| . | Randomized patients . | |||||
|---|---|---|---|---|---|---|
| 0.25 mg/kg AB023 (n = 8) . | 0.5 mg/kg AB023 (n = 8) . | Placebo (n = 8) . | ||||
| Predose* . | Postdose† . | Predose* . | Postdose† . | Predose* . | Postdose† . | |
| <15 min | 24/24 (100%) | 24/24 (100%) | 19/24 (79.2%) | 19/24 (79.2%) | 22/24 (92%) | 21/24 (87.5%) |
| ≥15 min | 0/24 (0%) | 0/24 (0%) | 5/24 (20.8%) | 5/24 (20.8%) | 2/24 (8%) | 3/24 (12.5%) |
| . | Randomized patients . | |||||
|---|---|---|---|---|---|---|
| 0.25 mg/kg AB023 (n = 8) . | 0.5 mg/kg AB023 (n = 8) . | Placebo (n = 8) . | ||||
| Predose* . | Postdose† . | Predose* . | Postdose† . | Predose* . | Postdose† . | |
| <15 min | 24/24 (100%) | 24/24 (100%) | 19/24 (79.2%) | 19/24 (79.2%) | 22/24 (92%) | 21/24 (87.5%) |
| ≥15 min | 0/24 (0%) | 0/24 (0%) | 5/24 (20.8%) | 5/24 (20.8%) | 2/24 (8%) | 3/24 (12.5%) |
Predose hemodialysis sessions include study day −7, −5, and −3 for a total of 24 measurements/group.
Postdose hemodialysis sessions include study day 1, 3, and 5 for a total of 24 measurements/group.
Efficacy
Thrombus accumulation and occlusive events
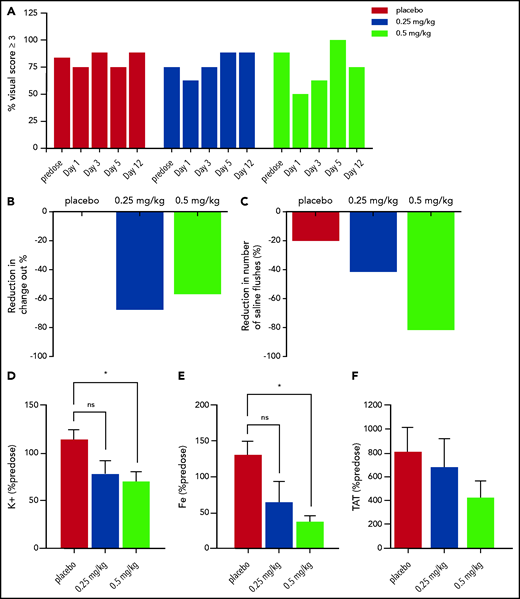
The average predose incidence of high-grade dialyzer clotting (score ≥3 corresponding to blood entrapment) was 83% for the placebo group (87.5%, 75%, and 87.5%, on study days −7, −5, and −3, respectively), 75% for the 0.25 mg/kg group (62.5%, 75%, and 87.5% on study days −7, −5, and −3, respectively), and 87.5% for the 0.5 mg/kg group (87.5% for all predose study days). The incidence of high-grade dialyzer clotting was 75%, 62.5%, and 50% on study day 1 in the placebo, 0.25 mg/kg, and 0.5 mg/kg cohorts respectively. On study day 3, the incidence of high-grade dialyzer clotting was 87.5%, 75%, and 62.5% in the placebo, 0.25 mg/kg, and 0.5 mg/kg cohorts, respectively. (Figure 2A). No observable changes in high-grade clotting in the venous chamber were observed in any of the groups (supplemental Figure 2).
Visual scores, dialyzer circuit change outs, saline flush events, and blood clot markers in the treatment arms. (A) The percent of severe clotting events (as defined as a visual score of ≥3) in each group (placebo, red bars; 0.25 mg/kg, blue bars; and 0.5 mg/kg, light green bars) within the hemodialysis cartridge. Predose data combine all predose hemodialysis days (study days −7, −5, and −3). (B) The bars represent the percent of occlusive events requiring change out of the hemodialysis circuit postdose (study days 1, 3, and 5 combined) compared with predose (study days −7, −5, and −3 combined). (C) Likewise, the bars represent the percent of hemodialysis circuit saline flushes required to maintain circuit patency postdose (study days 1, 3, and 5 combined) compared with predose (study days −7, −5, and −3 combined). (D-E) AB023 significantly reduces blood clot markers within the hemodialysis cartridge. AB023 (0.5 mg/kg) significantly reduced potassium (D) and iron (E) retained within the hemodialysis cartridge due to blood clotting on study day 1 compared with placebo. (F) AB023 may limit systemic increases in TAT after hemodialysis sessions. Predose samples were averaged, and shown is the percentage of predose potassium, iron, and TAT concentrations. n = 8 for placebo, n = 7 for 0.25 mg/kg, and 0.5 mg/kg potassium and iron groups (dialyzers were damaged upon receipt). Data represent mean ± SEM. *P < .05.
Visual scores, dialyzer circuit change outs, saline flush events, and blood clot markers in the treatment arms. (A) The percent of severe clotting events (as defined as a visual score of ≥3) in each group (placebo, red bars; 0.25 mg/kg, blue bars; and 0.5 mg/kg, light green bars) within the hemodialysis cartridge. Predose data combine all predose hemodialysis days (study days −7, −5, and −3). (B) The bars represent the percent of occlusive events requiring change out of the hemodialysis circuit postdose (study days 1, 3, and 5 combined) compared with predose (study days −7, −5, and −3 combined). (C) Likewise, the bars represent the percent of hemodialysis circuit saline flushes required to maintain circuit patency postdose (study days 1, 3, and 5 combined) compared with predose (study days −7, −5, and −3 combined). (D-E) AB023 significantly reduces blood clot markers within the hemodialysis cartridge. AB023 (0.5 mg/kg) significantly reduced potassium (D) and iron (E) retained within the hemodialysis cartridge due to blood clotting on study day 1 compared with placebo. (F) AB023 may limit systemic increases in TAT after hemodialysis sessions. Predose samples were averaged, and shown is the percentage of predose potassium, iron, and TAT concentrations. n = 8 for placebo, n = 7 for 0.25 mg/kg, and 0.5 mg/kg potassium and iron groups (dialyzers were damaged upon receipt). Data represent mean ± SEM. *P < .05.
Comparing pretreatment hemodialysis sessions (days −7, −5, and −3) to postdose hemodialysis sessions (days 1, 3, and 5), the incidence of occlusive events requiring circuit exchange went from 12.5% predose to 4% after 0.25 mg/kg AB023 (from 3/24 events to 1/24 events) and from 29% predose to 12.5% after 0.5 mg/kg AB023 (from 7/24 events to 3/24 events). The number of occlusive events was very low and numerically unchanged in the placebo arm (from 1/24 events to 1/24 events) (Figure 2B). It should be noted that the rate of clotting in the predose sessions that required dialyzer change out was not uniform across groups, with 4%, 12.5%, and 29% of predose hemodialysis events resulting in change out for the placebo, 0.25 mg/kg, and 0.5 mg/kg groups, respectively.
In dialyzers from the intention-to-treat population, potassium concentration, normalized to predose levels, on study day 1 was significantly lower after 0.5 mg/kg AB023 compared with placebo as measured by ICP-MS (Figure 2D). Similarly, iron concentration normalized to predose levels on study day 1 was significantly lower after 0.5 mg/kg of AB023 compared with placebo (Figure 2E). Since potassium and iron originated predominantly from trapped blood cells, the results are consistent with reduction in trapped blood volume in the hollow fibers after AB023 administration.
Saline flushes
Comparing predose hemodialysis sessions (days −7, −5, and −3) to postdose hemodialysis sessions (days 1, 3, and 5), the number of saline flushes needed to maintain circuit patency went from 27 total saline flushes predose to 15 total saline flushes postdose in the 0.25 mg/kg AB023 group and from 34 total saline flushes predose to 5 postdose in the 0.5 mg/kg AB023 group (Figure 2C). In the placebo group, there were 5 total saline flushes predose and 4 postdose.
TAT levels
To assess thrombin generation during hemodialysis sessions, systemic TAT levels were measured in plasma samples collected on study day 1 before dosing and at the end of the hemodialysis session (4 hours postdose). Increases in TAT levels were attenuated by AB023 in a dose-dependent manner (Figure 2F). Baseline TAT values were 11.3 ± 5.3, 6.74 ± 1.0, and 15.6 ± 6.9 ng/mL for the placebo, 0.25, and 0.5 mg/kg groups, respectively (mean ± SEM). Systemic TAT levels increased approximately eightfold following hemodialysis sessions compared with baseline in the placebo group. Following treatment with AB023, TAT levels increased approximately sixfold and approximately fourfold in the 0.25 mg/kg and 0.5 mg/kg groups, respectively.
Markers of dialysis efficiency
BUN and potassium were measured pre- and post-hemodialysis. The URR and Kt/V were calculated from the pre- and post-BUN values. Overall, mean BUN and potassium values decreased following hemodialysis (supplemental Table 2). The BUN and potassium clearance post-AB023 dosing increased compared with predose clearance within the group, and there was no difference in BUN and potassium clearance compared with placebo. There was no difference in mean URR or Kt/V values between treatments.
Inflammatory biomarker analysis
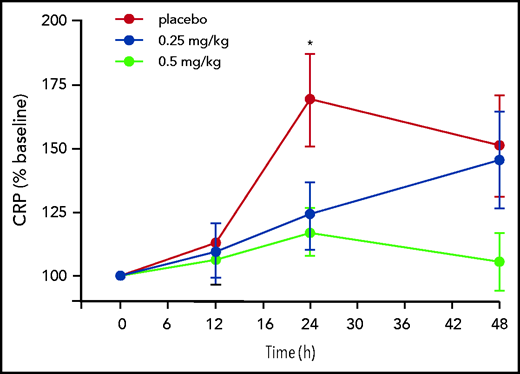
CRP plasma concentrations increased in the placebo grouped by >50% 24 hours after hemodialysis and remained elevated for 48 hours (Figure 3). AB023 significantly blunted the CRP increase at 24 hours, and CRP levels trended lower in the 0.5 mg/kg group at 48 hours after initiation of hemodialysis and dosing (Figure 3). AB023 had no statistically significant effect on IL-6 plasma concentrations (supplemental Figure 3).
AB023 blunts dialysis associated plasma CRP generation. Time course of plasma CRP on study day 1 predose through 48 hours postdose expressed as the percentage of control of baseline (predose) in the placebo group (red circles), the 0.25 mg/kg group (blue circles), and the 0.5 mg/kg group (light green circles). CRP induction at 24 hours postdose is significantly blunted in the 0.5 mg/kg group compared with placebo. Data are mean ± SEM. *P < .05 for the 0.5 mg/kg group compared with placebo.
AB023 blunts dialysis associated plasma CRP generation. Time course of plasma CRP on study day 1 predose through 48 hours postdose expressed as the percentage of control of baseline (predose) in the placebo group (red circles), the 0.25 mg/kg group (blue circles), and the 0.5 mg/kg group (light green circles). CRP induction at 24 hours postdose is significantly blunted in the 0.5 mg/kg group compared with placebo. Data are mean ± SEM. *P < .05 for the 0.5 mg/kg group compared with placebo.
PD and PK analysis
AB023 (either 0.25 or 0.5 mg/kg) prolonged the aPTT in all subjects (Figure 4A-B). Baseline aPTT values were 39.1 ± 1.5, 39.1 ± 2.8, and 42.2 ± 1.7 seconds in the placebo, AB023 0.25 mg/kg, and AB023 0.5 mg/kg groups, respectively (mean ± SEM). The maximum increase in aPTT (2.3-fold) was observed 3 to 4 hours after administration of AB023 on study day 1, and the mean aPTT values were 90.9 ± 7.6 and 96.0 ± 4.1 seconds in the AB023 0.25 mg/kg and 0.5 mg/kg dose groups, respectively. aPTT values decreased more rapidly in the 0.25 mg/kg AB023 group than in the 0.5 mg/kg AB023 group. An anticoagulant effect was observed for at least 3 and 5 days after 0.25 or 0.5 mg/kg AB023, respectively. By 24 hours postdose, the mean aPTT values were 69.8 ± 7.7 (1.6-fold increase over baseline) and 89.6 ± 3.6 seconds (2.2-fold increase over baseline) in the AB023 0.25 and 0.5 mg/kg dose groups, respectively. PT changes were not significant (Figure 4C).
PD and PK parameters from all subjects from the AB023 phase 2 clinical trial. Two cohorts were administered a single dose of AB023 (cohort 1, 0.25 mg/kg [n = 8, blue circles]; cohort 2, 0.5 mg/kg [n = 8, light green circles]) or placebo (all placebo-dosed subjects from both cohorts are grouped together [n = 8, red circles]). (A) aPTT for all groups from study day 1 predose (represented as 0 hours on the x-axis) until the end of the trial in hours. Arrowheads show study days where hemodialysis took place. (B) The aPTT data from panel A are expanded to show the first 24 hours postdose on study day 1 so that the data from the early postdose time points can be more clearly seen. (C) PT time from all subjects predose (study days −7, −5, and −3) and postdose (study days 1, 3, 5, and 12). There was no statistical difference in PT found in any cohort compared with placebo. (D) Mean AB023 plasma concentrations after a single IV dose. Plasma concentration of AB023 from both cohorts after a single injection of AB023 (0.25 mg/kg, blue circles; 0.5 mg/kg, light green circles; n = 8 for each dose level). Data represent mean ± SEM.
PD and PK parameters from all subjects from the AB023 phase 2 clinical trial. Two cohorts were administered a single dose of AB023 (cohort 1, 0.25 mg/kg [n = 8, blue circles]; cohort 2, 0.5 mg/kg [n = 8, light green circles]) or placebo (all placebo-dosed subjects from both cohorts are grouped together [n = 8, red circles]). (A) aPTT for all groups from study day 1 predose (represented as 0 hours on the x-axis) until the end of the trial in hours. Arrowheads show study days where hemodialysis took place. (B) The aPTT data from panel A are expanded to show the first 24 hours postdose on study day 1 so that the data from the early postdose time points can be more clearly seen. (C) PT time from all subjects predose (study days −7, −5, and −3) and postdose (study days 1, 3, 5, and 12). There was no statistical difference in PT found in any cohort compared with placebo. (D) Mean AB023 plasma concentrations after a single IV dose. Plasma concentration of AB023 from both cohorts after a single injection of AB023 (0.25 mg/kg, blue circles; 0.5 mg/kg, light green circles; n = 8 for each dose level). Data represent mean ± SEM.
Mean plasma AB023 concentration–time profiles following a single bolus injection of 0.25 and 0.5 mg/kg AB023 are presented on a linear scale in Figure 4D. Median times to maximum concentrations were between 0.167 and 0.750 hours. Plasma AB023 remained quantifiable for 48 hours after the 0.25 mg/kg dose and for 120 hours after the 0.5 mg/kg dose. Mean plasma AB023 concentrations were higher after the 0.5 mg/kg dose than after the 0.25 mg/kg dose. The summary of plasma AB023 PK parameters is presented in supplemental Table 3. Exposure of plasma AB023 from 0.25 mg/kg to 0.5 mg/kg increased in a greater than dose-proportional manner. Mean clearance decreased with increasing dose, and mean half-life appeared to increase from 11.1 to 27.1 hours with the increase in AB023 dose from 0.25 to 0.5 mg/kg.
Discussion
AB023 was well tolerated, without significant adverse events, in this small group of medically complex ESRD patients. Our exploratory efficacy end points showed a reduced incidence of occlusive events requiring hemodialysis circuit exchange, a decrease in circuit flushes required to maintain patency, less visually observed and scored dialyzer clotting, and lower accumulation of blood clot–derived potassium and iron within the dialyzer in AB023-treated patients when compared with their own heparin-free hemodialysis sessions. These data suggest that AB023 may be useful for safely reducing dialyzer clotting during heparin-free hemodialysis.
Despite erythropoietin and iron supplementation, most ESRD patients on chronic hemodialysis suffer from clinically relevant anemia, in part due to frequent blood loss from clotting in the hemodialyzer despite prophylactic heparinization.23 Even though hemodialyzers have been improved for biocompatibility, the problem of chronic blood loss may be aggravated in the setting of heparin-free dialysis. Moreover, repeated exposure of blood to extracorporeal medical device components can promote bleeding due to coagulation factor consumption, shear-mediated acquired von Willebrand disease, and platelet consumption and dysfunction.24-27 Cardiovascular events, including heart attack, stroke, or venous thromboembolism, are leading causes of death in ESRD patients, and hemodialysis-associated inflammation may contribute to the poor prognosis.28,29 Loss of dialysis access site patency due to thrombosis can further complicate treatment.30 A strategy to concurrently mitigate blood loss, thrombosis risk, and inflammation in ESRD patients on heparin-free dialysis is therefore highly desirable and remains unsolved by our currently available alternative anticoagulation armamentarium.
This trial supports the hypothesis that blood entrapment and clotting in hemodialyzers is promoted by contact system activation and that inhibiting FXII-driven FXI activation with AB023 reduces clotting within medical devices. Other FXI-targeting drugs in development inhibit FXIa or FXI activation or reduce FXI levels. While the target of AB023 is FXI, it uniquely appears to inhibit prothrombotic contact activation of FXI without inhibiting hemostatic FXI activation by thrombin. Indeed, FXIa can still be generated by tissue factor pathway–derived thrombin in the presence of AB023,11,15 and the resulting FXIa is not inhibited from activating coagulation FIX that can drive hemostatic thrombin generation. This unique mechanism of action selectively inhibits FXI activation by FXIIa, thereby effectively acting like a FXIIa inhibitor. Targeting FXII, FXI, and FXIIa-mediated FXI activation has reduced experimental thrombus development in various animal models.9-12 However, while FXI deficient or inhibited humans display a mild to moderate bleeding diathesis, human or animal FXII deficiency is asymptomatic.13,14 These data and observations suggest that AB023, even if overdosed, would be more likely to emulate the phenotype of hereditary FXII deficiency, which has no known bleeding diathesis, as compared with potent direct FXI/FXIa inhibitors, which would likely emulate the bleeding phenotype of hereditary FXI deficiency.
Cardiovascular events are a major cause of morbidity and mortality in hemodialysis patients, and chronic inflammation contributes to the progression of atherosclerosis. Indeed, CRP levels correlate with survival in hemodialysis patients.31,32 Here, we observed an attenuation of a modest hemodialysis-induced temporal serum CRP elevation after treatment with AB023, but the mechanism and relevance of this effect remain unclear. IL-6 stimulates CRP production,33,34 but AB023 had no significant effects on plasma IL-6 concentrations, which all remained within normal range. It is unlikely that the presence of AB023 interfered with the CRP detection, since samples with the highest AB023 concentrations did not consistently demonstrate lower CRP values, although this was not specifically assessed. Nonclinical studies from our group showed that AB023 (also known as 3G3) or its murine analog (14E11) can improve survival and reduce circulating markers of systemic inflammation in various animal models.35-39 Should chronic inflammation in ESRD patients contribute to their accelerated morbidity and mortality, it is tantalizing to consider the ramification of mitigating extracorporeal organ support device–associated inflammation with AB023.
Several studies suggest that heparins also have anti-inflammatory effects, although controlled outcome studies demonstrating the benefits of such effects have not yet been conducted in ESRD.40 For example, UFH and low-molecular-weight heparin (LMWH) exhibit similar anti-inflammatory effects in vitro.41 However, in a study comparing UFH with LMWH for hemodialysis, only LMWH reduced inflammatory markers, such as CRP.42 Likewise, in a murine model of deep-vein thrombosis, LMWH reduced inflammatory markers, whereas UFH did not.43 While UFH remains the preferred anticoagulant for use during hemodialysis of most ESRD patients, a safe alternative anticoagulant that could reduce both intradialyzer clotting and detrimental inflammation is needed for those ESRD patients who cannot tolerate heparins.2,44
This study was not intended to investigate AB023 in comparison with or in addition to heparin but aimed to assess the effects of AB023 during heparin-free dialysis, which is indicated and necessary in the fraction of the ESRD patient population on chronic hemodialysis that cannot tolerate heparin. Our conclusions are limited by the modest patient sample size yet enhanced by the trial design of comparing predose data to results gathered while AB023 was at saturating concentrations in the circulation. The number of dialysis sessions evaluated during this trial and the methods used to evaluate efficacy end points temper the trial size limitation. The heterogeneous patient population and small sample size likely explain the predose clotting differences between the placebo and the AB023 arms, and only larger-powered studies could address the above-mentioned limitations.
In summary, we found that a single dose of AB023 was well tolerated and reduced intradialyzer clotting during heparin-free hemodialysis in a group of ESRD patients. These compelling data support further definitive clinical trials targeting contact activation of blood in heparin-intolerant ESRD patients, including possible comparisons of AB023 to other accepted pharmacological approaches.
Acknowledgments
The authors of this work have been supported by grants from the National Institutes of Health (National Heart, Lung, and Blood Institute: HL106919, HL144113, HL101972, HL151367, and S10RR025512) and a seed grant from Oregon Health & Science University to the Elemental Analysis Core.
Authorship
Contribution: C.U.L., E.I.T., and A.G. conceived and designed the study; C.U.L., E.I.T., J.J.S., and A.G. oversaw the study; C.U.L., N.G.V., M.W., and B.D.M. performed ELISAs; M.R. performed ICP-MS analysis; and all authors participated in writing the manuscript, verified and interpreted the data, made the decision to submit the manuscript for publication, and vouch for the accuracy of the analyses.
Conflict-of-interest disclosure: C.U.L., N.G.V., M.W., B.D.M., E.I.T., and A.G. are employees of Aronora, and they, as well as Oregon Health & Science University, may have a financial interest in the results of this study. J.J.S. is a medical consultant for Aronora. The remaining authors declare no competing financial interests.
Correspondence: Christina U. Lorentz, Aronora, 1818 SW 4th Ave, Suite 102, Portland, OR 97201; e-mail: christina.lorentz@aronorabio.com.
Deidentified individual participant data underlying the reported results will be made available 3 months after publication for a period of 5 years. Proposals for access should be sent to christina.lorentz@aronorabio.com. The study protocol is included as a data supplement available with the online version of this article.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
C.U.L. and E.I.T. contributed equally to this study.

![PD and PK parameters from all subjects from the AB023 phase 2 clinical trial. Two cohorts were administered a single dose of AB023 (cohort 1, 0.25 mg/kg [n = 8, blue circles]; cohort 2, 0.5 mg/kg [n = 8, light green circles]) or placebo (all placebo-dosed subjects from both cohorts are grouped together [n = 8, red circles]). (A) aPTT for all groups from study day 1 predose (represented as 0 hours on the x-axis) until the end of the trial in hours. Arrowheads show study days where hemodialysis took place. (B) The aPTT data from panel A are expanded to show the first 24 hours postdose on study day 1 so that the data from the early postdose time points can be more clearly seen. (C) PT time from all subjects predose (study days −7, −5, and −3) and postdose (study days 1, 3, 5, and 12). There was no statistical difference in PT found in any cohort compared with placebo. (D) Mean AB023 plasma concentrations after a single IV dose. Plasma concentration of AB023 from both cohorts after a single injection of AB023 (0.25 mg/kg, blue circles; 0.5 mg/kg, light green circles; n = 8 for each dose level). Data represent mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/22/10.1182_blood.2021011725/5/m_bloodbld2021011725f4a.png?Expires=1769081174&Signature=QgspaKym5i4SOreZnn1Fu1NQS3pzwQM0bZSBbiKi9POcwHwKr0uV42MxEeLm~EL~yjuKt826kSDNy~JPhTzqpZccHozDgdmnmYL6wufBuq1Pna0fjs~RmTGJGHglQoBon9LAv6b0MVgRx55BybY5l7vhYMKCBLzbBf~2Mllch1ucmdGqc43rrTJiaHGb0aLkrtBzQC7xhMFOv1VOzi~WeCnwbD5MJ6hYcDbMhb6VVojseRxqgguye-BDRkvTBAMtV0RClyBoTpuayW61eKFZQL77BzMr29lWT~nOR5tTKaVgQQ3Koc-9r-lfUWgjkjoZqV5UMokK3ov3z2guu~Tn9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![PD and PK parameters from all subjects from the AB023 phase 2 clinical trial. Two cohorts were administered a single dose of AB023 (cohort 1, 0.25 mg/kg [n = 8, blue circles]; cohort 2, 0.5 mg/kg [n = 8, light green circles]) or placebo (all placebo-dosed subjects from both cohorts are grouped together [n = 8, red circles]). (A) aPTT for all groups from study day 1 predose (represented as 0 hours on the x-axis) until the end of the trial in hours. Arrowheads show study days where hemodialysis took place. (B) The aPTT data from panel A are expanded to show the first 24 hours postdose on study day 1 so that the data from the early postdose time points can be more clearly seen. (C) PT time from all subjects predose (study days −7, −5, and −3) and postdose (study days 1, 3, 5, and 12). There was no statistical difference in PT found in any cohort compared with placebo. (D) Mean AB023 plasma concentrations after a single IV dose. Plasma concentration of AB023 from both cohorts after a single injection of AB023 (0.25 mg/kg, blue circles; 0.5 mg/kg, light green circles; n = 8 for each dose level). Data represent mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/22/10.1182_blood.2021011725/5/m_bloodbld2021011725f4b.png?Expires=1769081174&Signature=jGiC0KOWCagC7s-n3OvXq6jO-Mq7UeSNHknr6jkGjPvjIJCDQ9yMgv8vHYTwOxBNaygkJ3BF~DiA3VazwYOL4aCxY38faoAnxO2LPYuHyAtG-mc94YSfK7~wW3s7tqZB8egzuizMMJQ244DNkIi7YVi5by1mz4VRJjh266QJv4IlGyo9KwHdMJI9zFWwAc7H5UtUtrX5YNA53CrwR4VOZ8Db1kDBQyIZFS8BnqXPY7xaCovXgxu4NxPFE3632ylTov58qwnt9bEeZUI1ODxYtrIr0AaK9LWeKfKmPZEQfrtlkMS2si0UZykY~GFscy9NynAxSWiqGowPFHRN3Z2O9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal