TO THE EDITOR:
We read with great interest the recent article published in Blood by Micklethwaite et al, “Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells.”1 In this article, the authors conducted a phase 1 clinical trial of piggyBac transposon–generated CD19-targeted chimeric antigen receptor (CAR) T cells in patients with relapsed or persistent B-cell lymphomas following allogeneic hematopoietic stem cell transplant. The authors describe the first 2 reported cases of T-cell lymphomas arising from CAR-modified T cells and perform detailed immunological, genomic, and transcriptomic analyses of these cells. Their work represents a highly important investigation into CAR T-cell safety.
The authors observed that the CAR lymphomas of both patients had insertions into introns 3 and 4 of BACH2, which were associated with mildly reduced expression of the gene. For several reasons, Micklethwaite et al argued that BACH2 inactivation may not contribute to lymphomagenesis. First, they note an absence of previous reports implicating BACH2 in T-cell lymphoma. Second, they contend that because BACH2 is already expressed at low levels in untransformed memory T cells, further downregulation of BACH2 levels by these insertions may not have a sufficient molecular effect to drive positive selection. Moreover, they note enrichment of retroviral insertion sites in the BACH2 gene locus. These data suggest that recurrent BACH2 insertions could thus result from the locus being a hotspot for insertion of foreign genetic material, rather than positive selection for BACH2-disrupted T cells.
However, in a paper recently published in Blood (Park et al2), we identify BACH2 as a putative tumor suppressor in T-cell malignancies for the first time. In our study, we performed whole-genome sequencing in patients with cutaneous T-cell lymphoma (CTCL). We used previously published algorithms to identify single nucleotide variants, copy number variants, and translocation events.3-5 The data, detailed methods, and description of samples are available in our paper. Our study was approved by the Northwestern University Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
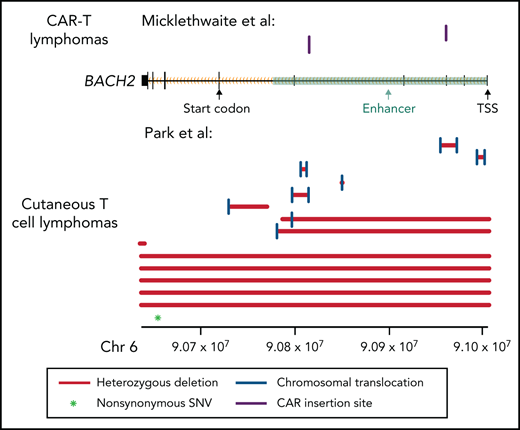
Multiple lines of evidence support a tumor suppressor role for BACH2 in CTCL. First, we performed an unbiased analysis for recurrent translocations that occur more often than expected by chance. This analysis identified BACH2 as the most significantly affected gene (8 samples, Padj = 1.3 × 10−7). These appear to be loss-of-function translocations, as they do not have a recurrent translocation partner. Moreover, the intronic breakpoint events occur in important BACH2 enhancers6,7between the start codon and the transcriptional start site (introns 1-5). These structural variants are predicted to inactivate gene expression by uncoupling the promoter from the protein-coding messenger RNA sequences (Figure 1). As is typical for tumor suppressors, there are also additional deleterious mutations. In this case, there are 6 additional samples with deletions that affect part of or the entire gene and an additional nonsynonymous point mutation. In total, we identified BACH2 alterations in 14% of CTCLs (Figure 1).
Mutation pattern consistent with a tumor suppressor role in BACH2. Alterations in the BACH2 gene locus in patients with CAR T-cell lymphomas (Micklethwaite et al1) and in CTCL (Park et al2). Black rectangles represent BACH2 exons. Coordinates shown use the hg19 genome build. SNV, single nucleotide variant; TSS, transcriptional start site.
Mutation pattern consistent with a tumor suppressor role in BACH2. Alterations in the BACH2 gene locus in patients with CAR T-cell lymphomas (Micklethwaite et al1) and in CTCL (Park et al2). Black rectangles represent BACH2 exons. Coordinates shown use the hg19 genome build. SNV, single nucleotide variant; TSS, transcriptional start site.
Like the breakpoints identified in CTCL, the CAR insertions observed by Micklethwaite et al occurred in enhancers in introns 3 and 4 (Figure 1). It is therefore not surprising that their insertion events lead to downregulation of gene expression in the malignant cells.1 In Micklethwaite et al, both patients had mild downregulation of BACH2 expression levels (patient 2: −0.17 log2 fold change; patient 8: −0.71 log2 fold change) in the CAR lymphoma as compared with the initial CAR product. Although others have observed that normal memory CD4+ T cells express less BACH2 than normal naive CD4+ T cells,8 modest decreases in BACH2 protein expression in T cells may be sufficient to cause clinical disease. Germline heterozygous BACH2 mutations reduce BACH2 protein expression in CD4+ T cells by only ∼25%. Nonetheless, they are sufficient to drive a clinical syndrome characterized by immunodeficiency and autoimmunity.9 The changes in T-cell immunophenotype can also be recapitulated by small interfering RNA reduction of BACH2 levels in healthy T cells and by haploinsufficiency of BACH2 in vivo in mice.9
BACH2 normally functions in T cells to limit expression of T-cell receptor–dependent transcriptional programs. It limits access of AP-1 transcription factors to their target DNA sequences.10 In Jurkat cells, BACH2 inhibits T-cell receptor–dependent AP-1–mediated induction of interleukin-2, a proproliferative cytokine.11 Of cancer relevance, the AP-1 pathway mediates drug resistance in CTCL12 and promotes growth and survival of other T-cell lymphomas.13
Disruption of T-cell lymphoma tumor suppressors may improve CAR T-cell fitness without inducing malignant transformation. For example, lentivirally produced CAR T cells have been observed to have clonal expansion of cells with inactivation of TET2, another T-cell lymphoma tumor suppressor.14,15 A phase 1 trial16 utilizing T-cell receptor–transgenic T cells with CRISPR/Cas9 editing of the T-cell lymphoma tumor suppressor PD-117 was recently reported and did not detect malignant transformation. Notably, the follow-up time was insufficient to exclude CAR malignancies occurring up to 11.5 months following infusion as reported in the Micklethwaite et al trial.1
CAR T-cell manufacturers must balance the risks and benefits of increasing efficacy and/or risk of malignant transformation. Thus, it is important to identify genetic events that can plausibly induce lymphomagenesis and functionally validate their effects. Additional functional studies are required to elucidate whether insertions in BACH2 had a causal impact on lymphomagenesis in the Micklethwaite et al trial. As noted by the authors, BACH2 is a hotspot for both recurrent retroviral and piggyBac insertions in primary human CD4+ T cells.18 However, it remains unclear whether the recurrence rate of insertions in the BACH2 locus was due to a preference for the insertion into this site or due to positive selection pressure for BACH2 mutant T cells. Last, because the clinical phenotypes of CTCL and CAR T-cell lymphomas differ, it will be important to address whether there are context-dependent effects of genes such as BACH2 or other tumor suppressors. If CAR T-cell lymphoma tumor suppressors are known, this information can be leveraged to screen genetically engineered T-cell products for those most likely to result in CAR product-derived cancers.
Acknowledgments
J.D. was supported, in part, by National Institutes of Health, National Cancer Institute grants T32 CA009560 and F30 CA265107. J.C. was supported, in part by the Leukemia and Lymphoma Society #1377-21, the American Cancer Society RSG-20-050-01, and the Damon Runyon Foundation DRCRF#CI-84-16.
Authorship
Contribution: J.D. and J.C. designed the project, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaehyuk Choi, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, 303 E. Superior St, Room 5-115, Chicago, IL 60611; e-mail: jaehyuk.choi@northwestern.edu.
All data supporting this analysis are available in the article by Park et al.2

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal