In this issue of Blood, Guo et al1 demonstrate by a combination of ex vivo human and in vivo mouse model experiments that platelets modulate CD8+ T-lymphocyte response during sepsis, causing diminished specific CD8+ T-cell counts and function (eg, cytokine release). In a murine model of polymicrobial sepsis, this platelet-mediated downregulation of CD8+ T lymphocytes is associated with reduced survival. This finding is surprising, because platelets induced an increase in CD8+ T-lymphocytes and cytokine release in a mouse model of cerebral malaria and in murine infections with lymphocytic choriomeningitis arenavirus,2,3 raising the possibility that the role of platelets differs, depending on whether the pathogen is a bacterium, a virus, or a parasite.
Using a cecal ligation and puncture polymicrobial sepsis model, the authors convincingly show that platelets upregulate expression of major histocompatibility complex class I (MHC-I, HLA-I) during sepsis. These MHC-I molecules on platelets seem to be responsible for downregulation of specific CD8+ T lymphocytes. In a platelet-lineage–specific mouse model where β2 microglobulin (required for MHC-I function) was knocked out, suppression of CD8+ T-lymphocyte count and function during polymicrobial sepsis was no longer observed.
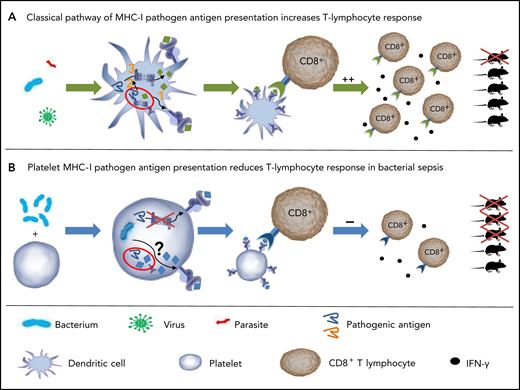
Model of antigen processing and presentation in DCs and platelets. (A) Classic pathways of MHC-I pathogen antigen processing in DCs. Exogenous antigens are internalized by DCs and subsequently processed (1) by the vacuolar pathway, by which the antigen is processed in vacuoles and then loaded on MHC-I; (2) by the phagosome-to-cytosol pathway, by which the antigen is taken up by vacuoles and then released into the cytoplasm. (3) Cytosolic antigens are processed by proteasomes and the resulting peptides loaded on MHC-I. Peptide-MHC-I complexes bind to specific T-cell receptors. T-cell proliferation and function are induced, improving survival of mice after infection. (B) Platelet MHC-I reduces CD8+ T-cell response in bacterial sepsis. Platelets internalize exogenous antigens. MHC-I is upregulated in platelets during sepsis. Antigen processing in platelets is independent of the platelet proteasome, at least for the model antigen OVA. Processed antigens are expressed by platelets through MHC-I. Interaction of platelet MHC-I with CD8+ T-lymphocytes reduces the count of CD8+ T-lymphocytes and cytokine release. In a polymicrobial mouse model, this interaction reduced survival.
Model of antigen processing and presentation in DCs and platelets. (A) Classic pathways of MHC-I pathogen antigen processing in DCs. Exogenous antigens are internalized by DCs and subsequently processed (1) by the vacuolar pathway, by which the antigen is processed in vacuoles and then loaded on MHC-I; (2) by the phagosome-to-cytosol pathway, by which the antigen is taken up by vacuoles and then released into the cytoplasm. (3) Cytosolic antigens are processed by proteasomes and the resulting peptides loaded on MHC-I. Peptide-MHC-I complexes bind to specific T-cell receptors. T-cell proliferation and function are induced, improving survival of mice after infection. (B) Platelet MHC-I reduces CD8+ T-cell response in bacterial sepsis. Platelets internalize exogenous antigens. MHC-I is upregulated in platelets during sepsis. Antigen processing in platelets is independent of the platelet proteasome, at least for the model antigen OVA. Processed antigens are expressed by platelets through MHC-I. Interaction of platelet MHC-I with CD8+ T-lymphocytes reduces the count of CD8+ T-lymphocytes and cytokine release. In a polymicrobial mouse model, this interaction reduced survival.
Recently, it has been shown that intact MHC class I molecules are particularly expressed by young platelets.4 Older platelets instead adsorb nonfunctional MHC-I from the plasma. The platelets that influence T lymphocytes are most likely the young platelet population. CD8+ T-lymphocyte function is not reduced in platelet-specific MHC-I–deficient mice, where platelets can still adsorb MHC-I shed from other cells. This raises interesting issues for transfusion medicine, particularly the question of the frequency of apheresis platelet donations by an individual donor, as platelet apheresis increases platelet turnover and the number of young platelets. This increase may change the potential of the transfused platelets to modulate T lymphocytes. An immunosuppressive effect is certainly not desirable in patients with sepsis requiring platelet transfusions for consumptive coagulopathy.
It is well known that platelets ingest exogenous proteins and particles. An important finding of Guo et al is that these proteins are processed by platelets to antigenic peptides and are subsequently loaded onto MHC-I molecules. Platelets express all catalytic subunits of the proteasome system and almost all other components of the MHC-I antigen-processing and -presentation pathway.5 In addition, platelet proteasome activity is enhanced in patients with sepsis.6 The importance of the proteasome system in platelets is also indicated by our observation that the platelet proteasome has surprisingly long stability of at least 7 days, whereas it has a much shorter half-life in epithelial cells and T lymphocytes.7 All these findings strongly indicate an important role of antigen processing by the proteasome in platelets in septic conditions.
In this regard, the present study had another unexpected finding. Measuring presentation of specific bacterial antigens in a model of polymicrobial sepsis is very challenging because of the high number of antigens presented. Guo et al used a fluorescently labeled ovalbumin (DQ-OVA) as a model antigen for analyses of antigen processing. Unexpectedly, the DQ-OVA epitope was still presented on platelet MHC-I when the proteasome complex was inhibited. This result raises the question of whether platelets typically use proteasome-independent pathway(s) for antigen processing or whether this finding is an exception related to the artificial nature of the OVA antigen.
A future challenge is to identify specific antigenic epitopes generated during sepsis and other conditions to determine the pathway by which they are processed in platelets. So far, antigen processing has been extensively studied in professional antigen-presenting cells, such as dendritic cells (DCs). Besides direct priming, CD8+ T lymphocytes can be induced by cross-presentation of antigens. For cross presentation, 2 pathways have been proposed in DCs: vacuolar and cytosolic (or phagosome-to-cytosol). In both pathways, proteasome complexes play an important role (see figure).8
Reduced T-lymphocyte counts have been reported in human sepsis and attributed to T-lymphocyte exhaustion related to chronic antigenic stimulation.9 Guo et al indicate that the platelet MHC-I peptide presentation to T lymphocytes may contribute to this reduction. Besides MHC-I, platelets express T-lymphocyte costimulation molecules such as CD86 (on human platelets only), CD84, CD40, and ICOSL, which enables platelets to activate naïve T lymphocytes.2 It would be interesting to see whether expression of costimulatory molecules on platelets is altered during sepsis and how they interplay with MHC-I–mediated antigen presentation.
It is also conceivable that MHC-I–mediated downregulation of CD8+ T cells and activity has beneficial effects in suppressing autoimmunity. This hypothesis becomes even more important by the fact that with 150 × 103 to 400 × 103 platelets/µL, the platelets outnumber the leukocytes by more than 1 order of magnitude. Furthermore, a rare recessive platelet disorder, gray platelet syndrome, is associated with increased susceptibility to autoimmune disorders.10 It would be intriguing to assess MHC-I expression and antigen processing in the gray platelet mouse model. Further insights will help in deciphering the functional relationship between platelet MHC-I and CD8+ T-lymphocyte activity in different settings and may even help to identify target structures for therapeutic intervention to modulate the immune response in sepsis, which is still a major cause of death in critically ill patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal