Abstract
Background
Clinical decision-making in patients with relapsed refractory multiple myeloma (RRMM) is challenging and identification of patients who can benefit the most from a given therapy is of critical importance. The International Myeloma Working Group (IMWG) index is one of the most robust scores to evaluate frailty, and it has been validated for patients with newly diagnosed MM. However, little is known on its applicability in the setting of RRMM and of its relationships with patient-reported health-related quality of life (HRQoL).
Objectives
The primary objective of this study was to investigate whether the IMWG frailty score is able to detect distinct patient-reported HRQoL profiles in the setting of RRMM. A secondary objective was to assess the prevalence of patient-reported clinically important symptoms by frailty groups (ie., fit, intermediate-fit, and frail).
Patients and Methods
This was an international (Italy and UK) prospective cohort observational study that consecutively enrolled patients from 30 centers. The patients were eligible if they had received at least 1 prior therapy (but no more than 5) and had RRMM according to IMWG criteria. An additional inclusion criterion at the time of study entry was the availability of all variables incorporated into the IMWG frailty score to allow classification of patients in the following three groups: fit, intermediate-fit and frail. Baseline assessment of HRQOL was mandatory to be included in this study and patients completed a set of well-validated measures including the EORTC QLQ-C30 and its myeloma module (QLQ-MY20). The scores from EORTC QLQ-C30 and MY20 questionnaires were summarized by means and standard deviations, overall and by frailty group. Unadjusted differences in mean scores were compared between frailty groups. We also estimated the adjusted mean differences in HRQoL scores of respectively fit and intermediate groups vs frail patients, using a multivariable linear regression model, adjusting for a number of key potential confounders including: sex, education, time since diagnosis, number of previous lines of therapy, previous transplantation, currently receiving therapy, myeloma status (refractory vs relapsed only) and type of MM at diagnosis (secretory vs else). We also assessed the prevalence of clinically important symptoms, based on previously published evidence-based thresholds, by IMWG frailty group.
Results
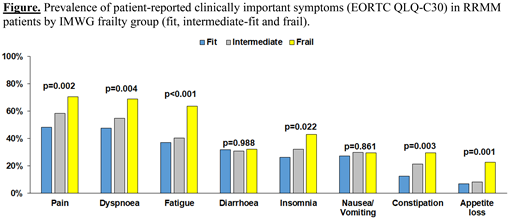
Overall, 365 RRMM patients were enrolled between November 2017 and December 2018. Median age was of 69.7 years (IQR, 62.7-75.0) and 296 had received at least two previous lines of therapy. According to the IMWG frailty score evaluation at study entry, 192 (53%), 85 (23%) and 88 (24%) patients were classified as fit, intermediate-fit and frail. Each group was associated with a clearly distinct patient-reported HRQoL profile with both fit and intermediate-fit groups reporting statistically and clinically meaningful better outcomes (ie., improved functional status and lower symptom burden) than frail patients in the majority of the scales from the EORTC QLQ-C30 and QLQ-MY20. For example, mean scores of the EORTC QLQ-C30 physical functioning scale were 71.2, 62.2, and 47.5 for fit, intermediate-fit and frail patients, respectively (p<0.001) (i.e., higher score indicates better functioning). Similarly, the mean score of the QLQ-MY-20 disease symptoms scale was 22.8, 25.9 and 35.9 for fit, intermediate-fit and frail patients, respectively (p<0.001) (i.e., higher score indicates higher symptom burden). Adjusted mean score differences (resulting from multivariable analysis) confirmed clinically relevant differences across most scales of the EORTC QLQ-C30 and QLQ-MY20. Additionally, the prevalence of patient-reported clinically important symptoms was statistically significant different across the three IMWG frailty groups with regard to pain (p=0.002), dyspnea (p=0.004), fatigue (p<0.001), insomnia (p=0.022) constipation (p=0.003) and appetite loss (p=0.001). Details are reported in Figure.
Conclusions:
In the setting of RRMM, the IMWG frailty score is able to detect clearly distinct patient-reported HRQoL profiles. Current findings may lay the groundwork for the development of a patient-centered frailty index, which also incorporates HRQoL data, to be used in patients with RRMM treated in real-life.
Efficace: Janssen: Consultancy; Abbvie: Consultancy, Other: Grants (to Institution); Amgen: Consultancy, Other: Grants (to Institution); Takeda: Consultancy. Gaidano: Incyte: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astrazeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Petrucci: BMS: Honoraria, Other: Advisory Board; Takeda: Honoraria, Other: Advisory Board; Amgen: Honoraria, Other: Advisory Board; GSK: Honoraria, Other: Advisory Board; Karyopharm: Honoraria, Other: Advisory Board; Janssen-Cilag: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board. Tafuri: Celgene: Research Funding; Roche: Research Funding; Novartis: Research Funding. Larocca: Amgen: Honoraria; Bristol-Myers Squibb: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; GSK: Honoraria; Takeda: Other: Advisory Board. Molica: Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Astrazeneca: Honoraria. Gozzetti: Janssen: Honoraria; AbbVie: Honoraria. Vignetti: Amgen: Consultancy, Honoraria; Incyte: Honoraria; Novartis: Honoraria. Cavo: Novartis: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau; Bristol-Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal