Abstract
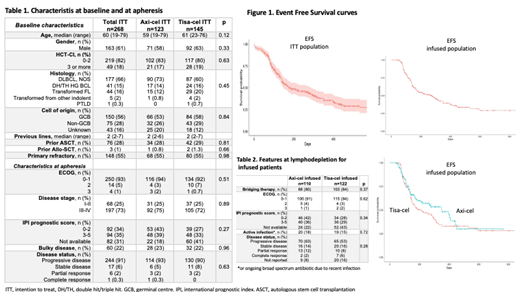
Introduction: Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are the two autologous anti-CD19 chimeric antigen receptors T cells commercially approved in Europe for relapsed/refractory (R/R) DLBCL. We performed a retrospective study to evaluate patients characteristics, efficacy and safety for axi-cel and tisa-cel in a large cohort of patients within the GETH-TC (Spanish Group of Stem Transplantation and Cell Therapy)-GELTAMO (Spanish Group of Lymphoma and Autologous Stem Cell Transplantation). Methods: Ten Spanish centers contributed data. Data were collected retrospectively from consecutive patients with DLBCL in whom apheresis was performed for axi-cel or tisa-cel treatment. CRS and ICANS were graded with the ASTCT consensus criteria. Response was assessed according to the Lugano criteria. Patients included had at least 30 days of follow-up. Results: A total of 268 patients with R/R DLBCL underwent apheresis for axi-cel (n=123) and tisacel (n=145) from Nov-2018 to May-2021, of which 232 (86%) received CART-cell infusion (n=110, 89% and n=122, 84%, respectively). Reasons for not undergoing infusion were progression in 10 cases, tumor lysis syndrome in 1, infection in 1, and CR after bridging therapy in 1 for the axi-cel cohort, and progression in 21 (4 after manufacture failure), psychiatric disorder in 1, and post-apheresis cerebral hemorrhage in 1 case for the tisa-cel cohort. Time between apheresis and infusion was 41 days (IQR 40-56) and 49 days (IQR 46-62) (p=0.006), respectively. Characteristics at baseline, apheresis and at lymphodepletion are summarized in Tables 1 and 2. Median age was 60 (range 19-79), 61% of patients were male, most of them treated for DLBCL NOS (66%). There were no significant differences between patients intended to be treated with axi-cel and tisa-cel. 82% of the infused patients received bridging therapy. At apheresis and at lymphodepletion ECOG performance status score was 0-1 in 93% and 91%, and 7 and 32 patients were in response, respectively. The overall response rates (ORRs) at 1 month and 3 months were 65% and 56%, respectively, with 34% and 45% achieving a complete response (CR), respectively. In the intention-to-treat analysis, with a median follow-up of 9 months (IQR 5-15), EFS and OS at 9 months was 44% (95%CI 38-51)(Figure 1) and 59% (95%CI 53-66) with a median EFS and OS of 6 months and 11 months, respectively. At lymphodepletion, characteristics of infused patients and disease were not significantly different between axi-cel and tisa-cel cohorts. Rates of CRS, CRS grade 3-4, ICANS, ICANS grade 3-4 were 89% and 71% (p=0.001), 10% and 7% (p=0.34), 42% and 16% (p=0.001), 20% and 4% (p=0.001) for the axi-cel and tisa-cel cohorts, respectively. ICU admission was needed in 25% and 15% of patients (p=0.06), respectively. Non-relapse mortality at days 100 were 2.5% and 1.4%, and at day 180, 5.4% and 2.2% (p=0.08) for the axi-cel and tisa-cel groups, respectively. With a median follow-up of 8 months for patients who received infusion with axi-cel and 12 months for patients infused with tisa-cel, EFS and OS at 12 months were 48% and 33% (p=0.33), and 53% and 50% (p=0.27), respectively, with a median EFS of 11 and 6 months and a median OS of 13 and 12 months, respectively. In the multivariate analysis, the only factor associated with poor PFS was having ECOG-PS ≥2 at lymphodepletion (HR13, p=0.03). No factors were identified as associated independently to OS. Factors associated to CRS grade 3-4 were IPI score 4-5 at lymphodepletion (OR 1.4, p=0.03) and ECOG-PS ≥2 at apheresis (OR 1.5, p=0.03). For ICANS grade 3-4, factors associated were the use of axi-cel (OR 8, p=0.04) and diagnosis of HG double/triple hit lymphoma (OR 9, p=0.022). Conclusions:This multicentric analysis describes one of the largest real-life cohorts of patients treated with axi-cel and tisa-cel for R/R aggressive B cell lymphoma in Europe. Results are comparable to those from the pivotal studies and other large real-life experiences. Up to now, patients included for one or other product in Spain do not differ significantly in terms of baseline characteristics, and within this setting, results are comparable between both products in terms of efficacy. The use of axi-cel was associated to higher rates of CRS and especially, severe forms of ICANS. However, mortality associated to toxicity were low and not significantly different between both cohorts.
Kwon: Novartis, Celgene, Gilead, Pfizer: Consultancy, Honoraria. Iacoboni: BMS/Celgene, Gilead, Novartis, Janssen, Roche: Honoraria. Reguera: Janssen, Kite/Gilead, Novartis: Speakers Bureau; BMS-Celgene, Novartis: Membership on an entity's Board of Directors or advisory committees. Lopez Corral: Gilead, Novartis: Consultancy, Honoraria. Guerreiro: Novartis, Gilead: Consultancy, Honoraria. Caballero: Novartis, Gilead: Honoraria. Ortiz-Maldonado: Kite, Novartis, BMS, Janssen: Honoraria. Sanchez: Janssen, Jazz Pharmaceuticals, Gilead, Novartis, Amgen: Consultancy. Sancho: Roche, Janssen, Celgene-BMS, Gilead, Novartis, Takeda: Honoraria, Speakers Bureau; Roche, Janssen, Celgene-BMS, Gilead, Novartis, Incyte, Beigene: Speakers Bureau. Bastos-Oreiro: Janssen: Honoraria, Speakers Bureau; F. Hoffmann-La Roche: Honoraria, Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS-Celgene: Honoraria, Speakers Bureau; Gilead: Honoraria; Kite: Speakers Bureau. Oarbeascoa: Gilead: Honoraria, Speakers Bureau. Mussetti: Gilead: Other: Unspecified, Research Funding; Novartis: Honoraria, Other: Unspecified; Takeda: Honoraria. Bailen: Gilead, Pfizer: Speakers Bureau. Martin Garcia-Sancho: Takeda: Honoraria; Novartis: Consultancy; Incyte: Consultancy; Celgene/BMS: Consultancy; Celgene: Honoraria, Other: travel; Roche: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Janssen: Honoraria, Research Funding; Servier: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Gilead: Consultancy, Honoraria; Morphosys: Consultancy; Kyowa Kirin: Consultancy; Clinigen: Consultancy; Eusa Pharma: Consultancy; Kern Pharma: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Barba: Amgen, Celgene, Gilead, Incyte, Jazz Pharmaceuticals, MSD, Novartis, Pfizer and Roche, Jazz Phar,aceuticals: Honoraria; Cqrlos III heqlth Institute, aSOCIACION espanola contra el cancer, PERIS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal