Abstract
Background: For relapsed and refractory multiple myeloma (RRMM), various clinical trials of B-cell maturation antigen-targeted chimeric antigen receptor (CAR) T-cell clinical trials are under way. For one such treatment, CT053, the Phase 1 investigator-initiated CG studies (NCT03380039, NCT03716856, NCT03302403) and the Phase 1 LUMMICAR STUDY 1 (NCT03975907) demonstrated deep and durable responses in heavily pretreated subjects with RRMM. Here we report integrated efficacy and safety of CT053 in Chinese subjects with RRMM based on high-risk factors.
Methods: All subjects had received ≥2 prior therapies, including at least an immunomodulatory drug and a proteasome inhibitor. Subjects also could have had exposure to an anti-CD38 antibody. All subjects were refractory to the last therapy per International Myeloma Working Group criteria. After lymphodepletion, subjects received a dose of 0.5 (n=1), 1.0 (n=4), 1.5 (n=32), or 1.8 (n=1) ×10 8 CAR+ T cells. We performed an integrated subgroup analyses of efficacy and safety in subjects stratified by high-risk characteristics, including extramedullary disease (EMD), high-risk cytogenetics [del(17p), t(4;14), t(14;16), t(14;20) and gain 1q], and International Staging System (ISS) stage III.
Results: A total of 38 subjects (LUMMICAR STUDY 1: n=14; CG studies: n=24) were treated with CT053 in Phase I studies in China. Of these, 31.6% subjects (12/38) had EMD, 47.4% (18/38) had high-risk cytogenetics, and 28.9% (11/38) had ISS stage III disease. After a median follow-up of 13.9 months, the objective response rate (ORR), complete response (CR) rate, median duration of response (mDOR) and median progression-free survival (mPFS) were 92.1%, 78.9%, 24.0 months [95% CI 13.3 months-not reached (NR)] and 22.7 months (95% CI 13.3 months-NR), respectively.
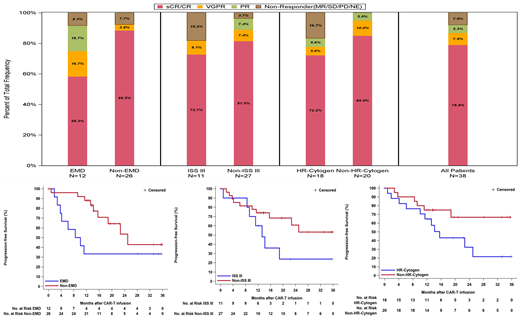
The ORRs for subjects with EMD, high-risk cytogenetics, and ISS stage III were 91.7% (95% CI 0.62-1.00), 83.3% (95% CI 0.59-0.96), and 81.8% (95% CI 0.48-0.98), respectively. A lower CR rate and poorer mDOR and mPFS were observed in subjects with EMD compared to subjects without EMD (58.3% vs 88.5%, 9.2 months vs 24.0 months, and 9.3 months vs 25.0 months, respectively). Although high-risk cytogenetics and ISS stage III disease at baseline did not substantially affect the CR rate, they seemed to negatively impact the DOR and PFS. The mDOR and mPFS were 14.8 months (95% CI 8.5 months-NR) and 15.6 months (95% CI 6.2-25.0 months) respectively for subjects with high-risk cytogenetics, and 13.3 months (95% CI 7.6 months-NR) and 13.3 months (95% CI 0.9 months-NR), respectively for subjects with ISS stage III. The median DOR and median PFS had not been reached in subjects without these two high-risk factors (Figure 1).
No dose limiting toxicity or CT053 treatment-related death occurred. The incidence of Grade 1/2 cytokine release syndrome (CRS) was 73.7%, and no ≥Grade 3 CRS occurred. Generally, CRS cases fully resolved after supportive care or tocilizumab. Only one subject with ISS stage III and EMD developed Grade 3 CAR T-cell-related encephalopathy syndrome (CRES), which fully resolved after methylprednisolone treatment. No other grade of CRES was observed in the studies.
Conclusions: The results demonstrate that CT053 represents a promising treatment option for subjects with RRMM, including those with high-risk disease. Phase 2 trials are actively enrolling.
LI: CARsgen Therapeutics Co. LtD: Current Employment, Current equity holder in publicly-traded company. Wang: CARsgen Therapeutics Corp: Current Employment. Zhao: CARsgen Therapeutics Corp: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal