Abstract
Translocation t(11;14) multiple myeloma (MM) is sensitive to the apoptosis-inducing drug venetoclax, yet the drug lacks FDA approval in MM. Selinexor is an inhibitor of nuclear export that is approved in relapsed/refractory MM. Here, we report that in patients with t(11;14) MM, the combined administration of venetoclax and selinexor was safe and resulted in clinically meaningful responses. This prompted preclinical studies to investigate synergism and molecular mechanisms of action. The combination was synergistic in t(11;14) MM cell lines and caused decreased levels of Cyclin D1 when given in combination as compared to single agents.
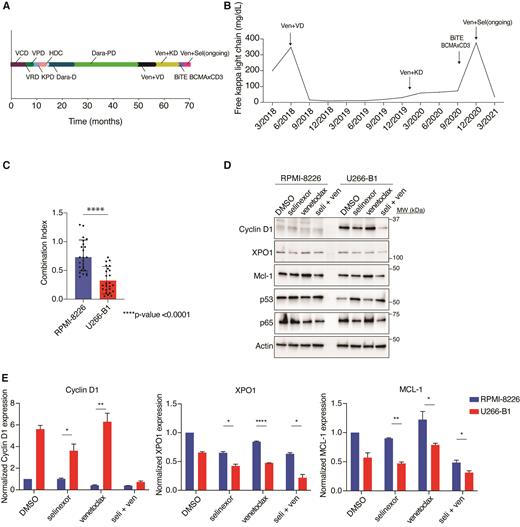
A 58-year-old African American man and an 81-year-old Caucasian woman with relapsed, refractory t(11;14) MM with CCND1-IGH fusion confirmed by FISH and progression of disease after multiple lines of therapy were treated with venetoclax based on previous data showing efficacy of venetoclax in t(11;14) MM. Both patients responded initially to venetoclax, however, developed resistance and progressive disease. The addition of selinexor recaptured responses (VGPR and MR, respectively) suggesting a beneficial effect of the combination over single agent venetoclax. The treatment course of the 58-year-old man is shown in Figure A and free kappa light chain response in Figure B.
Based on these observations, we hypothesized that selinexor with venetoclax was synergistic in patients bearing the t(11;14) translocation. We therefore studied the combination in MM cell lines with (U266-B1, KMS-12-BM, SK-MM2), and without (RPMI-8226, LP-1, OPM-2) t(11;14) translocations. We performed cell viability assays in increasing concentrations of selinexor, venetoclax, and a combination of the two drugs at 72 hours. Synergy was analyzed via the Bliss independence model using Synergy Finder software as well as via the Chou-Talalay method by using CompuSyn software. Average Bliss model synergy scores were -0.5 in non-t(11;14) and 10.2 in t(11;14) MM cells (>10 indicates synergistic effects and <-10 indicates antagonistic drug effects). Combination index (CI) values <1 are synergistic, CI=1 are additive, and >1 are antagonistic. Cell lines that possessed t(11;14) were more sensitive to the drug combination and showed enhanced synergy in those cell lines bearing the CCND1-IGH translocation (Figure C).
To better understand molecular mechanisms underlying the observed synergistic effect, we performed western blot analysis in these six cell lines, treating with selinexor (200nM), venetoclax (1μM), the combination, or DMSO control for 24 hours. We measured protein expression with antibodies against Cyclin D1, which is overexpressed in t(11;14) and a cargo of XPO1. Additionally, we measured levels of XPO1, p53, MCL-1, and p65, which we have previously shown to be altered by selinexor treatment (Figure D). We confirmed Cyclin D1 overexpression in t(11;14) cells lines but not in non-t(11;14) cells. Cyclin D1 levels decreased with selinexor, and the reduction was enhanced by adding venetoclax. Similarly, XPO1 levels decreased to a further degree in t(11;14) cell lines with the combination when compared to either drug alone. There was no difference in XPO1 reduction with the treatment combination in non-t(11;14) cell lines. P53 levels increased as a result of selinexor and combination treatment, and the combination also caused decreased levels of p65 in cell lines with and without t(11;14). Venetoclax upregulated MCL-1, but this was mitigated with the addition of selinexor. These effects were statistically more significant in t(11;14) cell lines (Figure E).
The combination of selinexor and venetoclax has shown preclinical synergy in other cancer types and is in Phase 1b clinical trials for relapsed, refractory non-Hodgkin lymphoma or acute myeloid leukemia (NCT03955783; NCT04607772). To our knowledge, this is the first report of patients with MM treated with the combination of selinexor and venetoclax. The mechanism behind the preferential synergy in t(11;14) MM is still under investigation; however, the result of our studies suggests a role for Cyclin D1, which is a cargo protein of XPO1. Additionally, while the effect of venetoclax on Cyclin D1 is not well defined, prior studies suggest the interplay between Cyclin D1, BCL2, and other anti- and pro-apoptotic proteins as having a role in oncogenesis. Based on our results, further clinical evaluation of this combination in MM is planned.
Bradley: AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Maura: OncLive: Honoraria; Medscape: Consultancy, Honoraria. Kazandjian: Arcellx: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Landgren: Janssen: Other: IDMC; Takeda: Other: IDMC; Celgene: Research Funding; Amgen: Honoraria; Janssen: Honoraria; Janssen: Research Funding; Amgen: Research Funding; GSK: Honoraria.
Venetoclax for myeloma is not yet FDA approved, but is used at clinician's discretion in patients who possess t(11;14) based upon the previous sub-group analysis of trials with venetoclax.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal