Abstract
Introduction: The benefit of direct oral anticoagulants (DOAC; Factor Xa Inhibitors [FXaI]) has been demonstrated in both clinical trials and real-world studies. However, 2% to 3.5% of DOAC-treated patients experience major bleeding annually, and this is associated with substantial morbidity, mortality, and the need for hospitalization. Therefore, reversal/hemostatic agents are used to control FXaI-related bleeding. The efficacy and safety profile of prothrombin complex concentrates (PCCs) as hemostatic agents in patients with FXaI-related bleeding requires further investigation.
Methods: The pivotal LEX-210 study (NCT04867837) is a Phase 3, multicenter, prospective, randomized, double-blinded, group-sequential, parallel-group, adaptive design study to demonstrate the hemostatic efficacy and safety of four-factor PCC (Octaplex®, Octapharma) in patients with acute major bleeding on DOAC therapy with FXaI. LEX-210 will include patients aged ≥18 years who have received or are believed to have received a dose of oral FXaI. Patients must have a baseline anti-factor Xa activity equivalent to at least 100 ng/mL according to the available test (e.g., chromogenic assay) and have acute major bleeding. Key exclusion criteria are bleeding that is immediately life-threatening and acute trauma for which reversal of DOAC therapy with FXaI alone would not be expected to control the bleeding event.
The study will enroll approximately 200 patients, with the aim to include at least 91 evaluable patients in each group. Patients will be randomized 1:1 to either of two study groups: low-dose 15 IU/kg body weight vs. high-dose 50 IU/kg body weight PCC. The primary objective of this study is to demonstrate superior hemostatic effectiveness of PCC dosed at 50 IU/kg vs. 15 IU/kg for emergency reversal of the anticoagulant effect of DOACs in patients with major bleeding associated with FXaI.
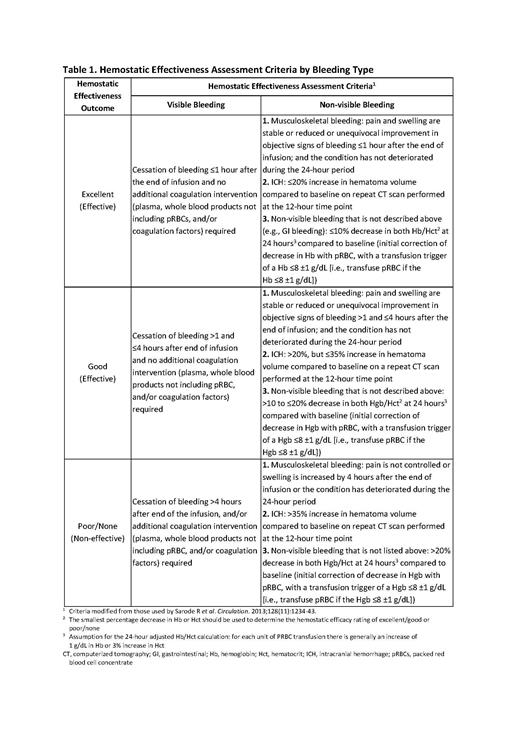
The primary endpoint of LEX-210 is the proportion of patients in whom PCC demonstrates hemostatic effectiveness, i.e., binary outcome of effective (rating of excellent or good) or non-effective (rating of poor or none) in management of major bleeding events within 24 hours after the start of initial management, as assessed by an Independent Data Monitoring and Endpoint Adjudication Committee according to predefined criteria modified from those used by Sarode et al., (see Table 1). Secondary endpoints are the change in endogenous thrombin potential as measured by thrombin generation assay from baseline to 1 hour after PCC administration, the 30-day event rate of thromboembolic events and all-cause mortality, the occurrence of adverse events, and vital signs and laboratory parameters.
Results: LEX-210 is planned to start in Q3 2021 and will be performed at approximately 60 sites in North America and Europe. Completion is expected by Q1 2024.
Conclusions: The LEX-210 study is designed to confirm the safety and hemostatic efficacy of PCC in the management of FXaI-related major bleeding, offering an effective alternative for the management of major bleeding events in these patients.
Sarode: Portola: Consultancy; CSL Behring: Consultancy; Octapharma: Consultancy; Cerus: Research Funding; Siemens: Research Funding. Maack: Octapharma: Current Employment. Solomon: Octapharma: Current Employment. Knaub: Octapharma: Current Employment. Schulman: Octapharma: Research Funding; Boehringer-Ingelheim: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal