Abstract
Introduction
Tafasitamab is a humanized, Fc-modified, anti-CD19 monoclonal antibody (mAb) shown to enhance antibody-dependent cellular cytotoxicity and phagocytosis. It is approved by the FDA under accelerated approval (July 2020) in combination with lenalidomide (LEN) for treatment of relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) in adult patients ineligible for autologous stem cell transplantation. To evaluate if newly diagnosed DLBCL patients would also benefit from this regimen, this Phase Ib study (First-MIND; NCT04134936) was designed to first assess the safety and tolerability of tafasitamab ± LEN in addition to rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) in newly diagnosed DLBCL patients.
Methods
Eligible patients were ≥18 years old, with treatment-naïve DLBCL, international prognostic index (IPI) 2-5 and Eastern Cooperative Oncology Group performance status (ECOG PS) 0-2. Patients with known double- or triple-hit and transformed lymphoma were excluded. Patients were randomized 1:1 to either six 21-day [D] cycles of R-CHOP (R-CHO, D1; P, D1-5) + tafasitamab (12 mg/kg IV, D1, 8, 15) (Arm A) or R-CHOP + tafasitamab + LEN (25 mg orally, D1-10) (Arm B). Granulocyte colony-stimulating factor and venous thromboembolism prophylaxis was mandatory. The primary safety endpoint was incidence of treatment-emergent adverse events (TEAEs); secondary endpoints included overall response rate (ORR), positron emission tomography (PET)-complete response (CR) rate, and partial response (PR) rate at end of treatment (EOT). Tumor measurements by PET/CT or PET/MRI at EOT were performed according to Lugano 2014 criteria within 6±2 weeks after Day 21 of the last treatment cycle the patient started.
Results
From December 2019 to August 2020, 83 patients were screened in nine countries across 34 sites in Europe and the US; 66 were randomized (Arm A, n=33; Arm B, n=33). Data cut-off for this analysis: 13 March 2021. Median age was 64.5 years (range 20-86). Many patients had high-risk disease: IPI 2: 24/66 (36.4%), IPI 3: 29/66 (43.9%), IPI 4: 11/66 (16.7%), IPI 5: 2/66 (3.0%); ECOG PS ≥2: 6/66 (9.1%). Most patients had Stage III/IV disease 62/66 (93.9%); 29/66 (43.9%) had bulky disease.
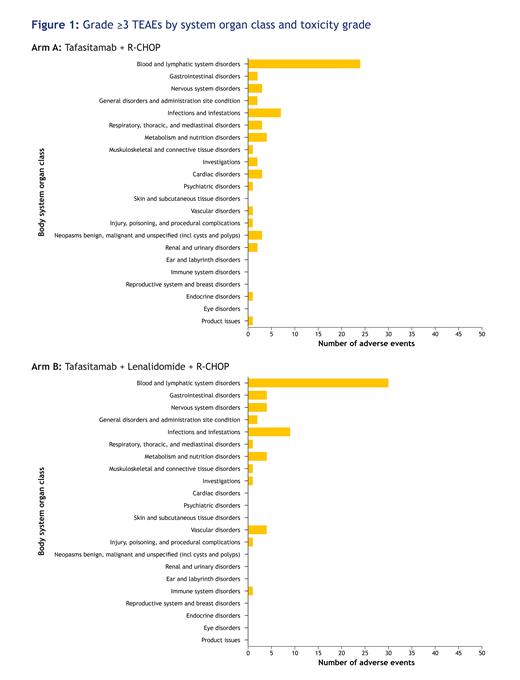
All patients experienced at least one TEAE. The most frequent Grade ≥3 TEAEs by system organ class were blood and lymphatic disorders (81.8% patients overall), experienced by 24 patients (72.7%) in Arm A and 30 patients (90.9%) in Arm B. Grade ≥3 neutropenia and thrombocytopenia occurred in 19/33 (57.6%) and 4/33 (12.1%) (Arm A), and 28/33 (84.8%) and 12/33 (36.4%) (Arm B) patients, respectively; Grade ≥3 febrile neutropenia was experienced by 6/33 (18.2%) patients in each arm. Grade ≥3 infusion-related reactions to both rituximab and tafasitamab occurred in no patients in Arm A and one patient (3.0%) in Arm B, and 7/33 (21.2%) (Arm A) and 9/33 (27.3%) (Arm B) patients had a Grade ≥3 infection and/or infestation (Figure 1). Serious TEAEs occurred in 14/33 (42.4%) patients in Arm A and 17/33 (51.5%) patients in Arm B. Two out of 33 (6.1%) and one out of 33 (3.0%) patients discontinued study treatment due to TEAEs in Arms A and B, respectively. There were four deaths unrelated to tafasitamab and/or LEN (COVID-19 pneumonia, sepsis, and urosepsis). The average relative dose intensity of R-CHOP in each cycle was maintained in both arms. ORR at EOT was observed in 25/33 patients (75.8%; 95% confidence interval [CI]: 57.7-88.9) in Arm A and 27/33 patients (81.8%; 95% CI: 64.5-93.0) in Arm B.
Conclusion
These data suggest that both regimens are tolerable and do not impair dosing and scheduling of R-CHOP. Toxicities were similar to those expected with R-CHOP with or without LEN. With tumor follow-up still ongoing, the ORR at EOT suggests that patients with treatment-naïve DLBCL may achieve clinically meaningful efficacy tafasitamab and LEN in addition to standard treatment. A Phase III, multicenter, randomized, double-blind, placebo-controlled trial will assess efficacy and safety of R-CHOP + tafasitamab + LEN vs R-CHOP in newly diagnosed patients with high intermediate and high risk DLBCL, and is currently recruiting (frontMIND study; NCT04824092).
Funding: MorphoSys AG.
Belada: Roche: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding; Gilead Sciences: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding; Janssen-Cilag: Consultancy, Research Funding; Takeda: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding; MorphoSys AG: Consultancy, Research Funding; Debiopharm Group: Consultancy; Pharmacyclics: Research Funding; Archigen Biotech: Research Funding; Reddys: Research Funding. André: Johnson & Johnson: Research Funding; Roche: Other: Travel/accomodation/expenses, Research Funding; Incyte: Consultancy; Gilead: Consultancy, Other: Travel/Accommodations/Expenses; Karyopharm: Consultancy; Bristol-Myers-Squibb: Consultancy, Other: Travel/Accommodations/Expenses; Takeda: Consultancy, Research Funding; Celgene: Other: Travel/accomodation/expenses; AbbVie: Other: Travel/accomodation/expenses. Pérez Persona: Amgen: Consultancy, Other: Support for attending meetings and/or travel, Speakers Bureau; BMS/Celgene: Consultancy, Other: Support for attending meetings and/or travel, Speakers Bureau; AbbVie: Other: Support for attending meetings and/or travel, Speakers Bureau; Takeda: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; AstraZeneca: Speakers Bureau; GSK: Consultancy; Incyte: Consultancy. Staber: Roche: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; MSD: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria. Trneny: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Amgen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Consultancy; 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Portola: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria. Brackertz: MorphoSys AG: Current Employment. Shah: MorphoSys AG: Current Employment. Sporchia: MorphoSys AG: Current Employment. Burke: MorphoSys: Consultancy; Adaptive Biotechnologies: Consultancy; Verastem: Consultancy; AstraZeneca: Consultancy; Bristol Myers Squibb: Consultancy; Kymera: Consultancy; X4 Pharmaceuticals: Consultancy; AbbVie: Consultancy; SeaGen: Consultancy, Speakers Bureau; Kura: Consultancy; Roche/Genentech: Consultancy; Epizyme: Consultancy; Beigene: Consultancy, Speakers Bureau. Nowakowski: Celgene, NanoString Technologies, MorphoSys: Research Funding; Celgene, MorphoSys, Genentech, Selvita, Debiopharm Group, Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Tafasitamab is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. In combination with lenalidomide (LEN), it received accelerated approval in July 2020 for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from lowâ€'grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT). Following FDA approval, we are now evaluating the safety and efficacy of tafasitamab in combination with LEN as an add-on to first-line therapy with R-CHOP in newly diagnosed patients with DLBCL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal