Abstract
Introduction: Therapy-related myeloid neoplasm (t-MN) is defined as the development of a myeloid neoplasm in the context of prior DNA damaging therapies. t-MN is an aggressive disease with no effective therapies. BCL2-inhibitor venetoclax (VEN) represents a novel treatment for acute myeloid leukemia (AML) patients including those with high-risk features. Available VEN studies included only a small portion of t-AML patients, and its efficacy in t-MDS is not known. Our aim was to study the efficacy and outcomes of VEN in t-MN.
Methods: Patients diagnosed with t-MN based on the WHO criteria who received VEN were identified. Minimal residual disease (MRD) assessment was performed in a subset of patients by multiparametric flow cytometry or molecular marker as available. Best response to VEN was divided into 3 categories: 1) MRD(-) complete remission (CR); 2) other CR [including complete morphological remission, MRD(+) CR, and CR with incomplete count recovery]; and 3) partial response/progressive disease/stable disease (PR/PD/SD). Progression-free (PFS) and overall survival (OS) were calculated from the time of initiation of VEN therapy using Kaplan-Meier analysis using Wilcoxon test. Logistic regression analysis was performed using Cox Proportional Hazard method. Statistical analysis was performed using JMP (v14.1, SAS Institute) and significance was defined as P<0.05.
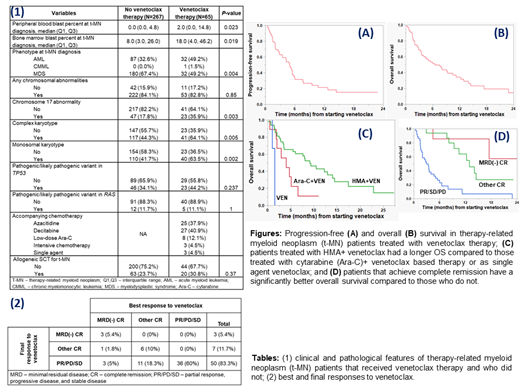
Results: Of 332 t-MN patients identified (248 at Mayo Clinic, 84 at the Central Adelaide Health Network), 65 (19.6%) received VEN. Clinical and pathological characteristics of patients that received VEN and did not receive VEN (non-VEN) were matched except: a higher proportion of VEN patients had t-AML (49.2% vs. 32.6%, P=0.004), higher peripheral (2% vs. 0%, P=0.02), and bone marrow blasts (18% vs. 8%, P=0.02). VEN patients had a higher proportion of abnormality of chromosome 17, complex karyotype, and monosomal karyotype compared to non-VEN patients. The proportion of patients with pathogenic/likely pathogenic variant (PV) in TP53 or RAS genes was not different. The proportion of patients receiving stem cell transplant for t-MN was not different (Table 1).
The indication for VEN was treatment naïve t-MN, relapsed/refractory (R/R) disease, and maintenance in 31 (47.7%), 32 (49.2%), and 2 (3.1%) patients respectively. Prior to VEN, patients received a median of 1 (range 0-4) lines of therapy. The median duration of VEN exposure was 3 cycles (IQR 1-4). VEN was administered with azacitidine, decitabine, low dose cytarabine, intensive chemotherapy, single agent, and >1 agents in 37.9%, 40.9%, 12.1%, 4.5%, 4.5%, and 2.9% respectively. The best and the final responses to VEN are shown in Table 2. Median PFS and OS from initiation of VEN was 5 months (IQR 2-9.5, Fig. A) and 6.5 months (2-14.5, Fig. B) respectively.
Female sex (vs. male, χ 2 9.6, P=0.03), VEN as the 1 st line of therapy (vs. other line of therapy, χ 2 8.3, P=0.02), and hypomethylating agent (HMA)-backbone (vs. other, χ 2 12.1, P=0.02) were associated with a significantly higher likelihood of achieving CR. Abnormality of chromosome 7 was associated with a shorter PFS (3.5 vs. 6 months, P=0.03) and OS (4.7 vs. 10.5 months, P=0.02). Using VEN as the 1 st line therapy was associated with an improved PFS (5.5 vs. 2.7 months, P<0.01) and a trend towards improved OS (9.3 vs. 5.1 months, P=0.06). Using HMA (n=51), Ara-C based (n=8), and single agent VEN (n=3) was associated with median PFS of 5.5, 4.2, and 1.5 months (P<0.01) and OS of 9.1, 4, and 1.5 months (P<0.01, Fig. C) respectively. Achieving MRD(-) CR (n=7), other CR (n=17), and PR/PD/SD (n=36) was associated with a median PFS of 7.7, 9.5, and 2 months (P<0.001) and median OS not reached, 12.9, and 2.9 months respectively (P<0.01, Fig. D). Finally, best response rate, PFS, and OS did not differ based on morphological diagnosis [t-MDS vs. t-AML] at diagnosis or bone marrow blast percent [<20% vs. ≥20%] at the start of VEN therapy. On multivariate analysis, only the best response remained an independent predictor of PFS and OS.
Conclusion: VEN induced remission, including MRD(-) remission, in a subset of patients. However, progression was noted in 83% patients with continued use, and PFS and OS were overall short. The morphological diagnosis of t-MDS/t-AML or blast percentage at the start of VEN therapy did not impact outcome. Earlier use of VEN, using HMA-based therapies, and achieving deeper responses were associated with improved outcomes.
Al-Kali: Novartis: Research Funding; Astex: Other: Research support to institution. Patnaik: StemLine: Research Funding; Kura Oncology: Research Funding. Wei: Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Macrogenics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tiong: Pfizer: Consultancy; Amgen: Speakers Bureau; Servier: Consultancy, Speakers Bureau. Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
venetoclax is not approved in the treatment of myelodysplastic syndrome.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal