Abstract
Introduction. The potential drug-drug interactions of midostaurin (M), particularly with CYP450 inhibitors, may impact the choice of antifungal (AF) prophylaxis and treatment in FLT3-positive (FLT3+) acute myeloid leukemia (AML) patients (pts). However, there are no supportive data to guide the choice of AF drugs in this subset of pts.
Aim. To evaluate the incidence of invasive fungal diseases (IFD) during induction and consolidation treatment of FLT3+ AML pts and to correlate it to the different AF prophylaxis strategies adopted.
Patients and Methods. Within the SEIFEM (Sorveglianza Epidemiologica Infezioni nelle Emopatie) Group, we planned a restrospective/prospective multicenter observational study enrolling all FLT3+ AML pts treated with chemotherapy (CHT)+M. IFD were classified as possible, probable and proven according to EORTC/MSG revised definitions (Donnelly JP et al, Clin Infect Dis 2020). Relationships between the type of AF prophylaxis, IFD development and AML outcome were evaluated.
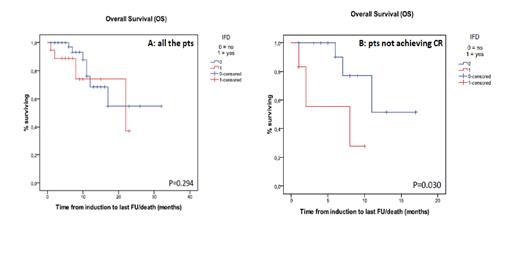
Results. As of 30 th June, 90 pts treated with CHT+M as induction/reinduction, consolidation or both, have been enrolled. M/F ratio was 35/55, median age 56y (range 18-78). FLT-ITD and FLT3-TKD mutations were detected in 73 and 17 pts, respectively, NPM-1 mutation in 51 (57%) pts. AML risk stratification according to ELN classification was available in 80 pts (24 low risk). A total of 216 CHT+M courses have been delivered (85 inductions, 8 reinductions and 135 consolidations). Overall, 25 IFD were reported: 19 during induction (22%), 2 during reinduction (25%) and 4 during consolidation (3%) given without AF prophylaxis in all the four. Sixty-five (72%) pts achieved complete remission (CR) after the induction. During induction, 11 pts did not receive mold-active AF prophylaxis and developed 4 (36%) IFD (3 possible and 1 probable aspergillosis), while 74 pts received mold-active AF prophylaxis and developed 15 (20%) IFD (8 possible and 4 probable aspergillosis, 3 candidemia). Incidence according to mold-active AF prophylaxis strategy was: 7/40 (17%) with posaconazole 300 mg/d, 5/13 (38%) with echinocandins (micafungin 50 mg/d or caspofungin 70>50 mg/d), 2/17 (12%) with posaconazole given for 7 days followed by micafungin or caspofungin, 1/2 with isavuconazole 200 mg/d, and 0/2 with L-AmB 50 mg every other day). A posaconazole-containing AF prophylaxis regimen was protective against IFD (9/57, 16%, vs 10/28, 36%, p=0.05), while a trend toward a higher incidence of IFD was observed in pts receiving echinocandins alone as AF prophylaxis (5/13, 38%, vs 14/72, 19%, p=0.15). IFD were more frequent in pts aged 60y or older (11/31, 35%, vs 8/54, 15%, p=0.034), while no correlations between IFD and gender, ELN classification, failure to achieve CR were observed. At multivariate analysis, age≥60y was confirmed as a risk factor for IFD (OR 3.021, CI 1.028-8.849), while receiving AF prophylaxis without posaconazole was of borderline significance (OR 2.817, CI 0.955-8.306). Three pts out of 40 on posaconazole prophylaxis received reduced dose of M (50 mg/d). M was discontinued in 12 pts during induction; of these, 6 (50%) did not achieve a CR. IFD was the main reason for M discontinuation in 6 pts; in the other 6, toxicity was responsible (QTc prolongation in 3, 1 during caspofungin, 1 during posaconazole and 1 out of AF prophylaxis; gastrointestinal toxicity in 2, 1 during posaconazole and 1 during caspofungin prophylaxis; liver toxicity in 1, during posaconazole prophylaxis). After a median follow-up of 5 months, overall survival (OS) was similar in pts with or without IFD (p=0.294), while in those not achieving CR after first induction it was significantly lower in pts developing IFD (p=0.03) (Fig. 1A and 1B).
Conclusions. IFD is still a frequent complication during AML induction treatment, also in patients receiving AF prophylaxis; it is associated with a frequent discontinuation of M and to a worsening of OS in patients not in CR after the first induction, probably contributing to a delay in the appropriate timing of the AML treatment. A posaconazole-containing prophylaxis was a better strategy against IFD occurrence, compared to echinocandins alone. M discontinuation for toxicity was a relatively rare event and no specific relationship with the type of AF prophylaxis ongoing was observed. A study evaluating plasma M levels during different prophylaxis strategies adopted is ongoing within the SEIFEM group.
Fracchiolla: Gilead: Honoraria, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rossi: Alexion: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Honoraria; Jazz: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees. Pagano: Gilead Sciences, MSD, Pfizer Pharmaceuticals, Astellas Pharma: Speakers Bureau; Gilead Science, MSD, Pfizer, Basilea, Janssen, Novartis, Jazz Pharmaceutical, Cidara: Membership on an entity's Board of Directors or advisory committees; Menarini: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal